2009
GRASP with Motion Compensation for DCE-MRI of the Abdomen1Center for Advanced Imaging Innovation and Research (CAI2R) and Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
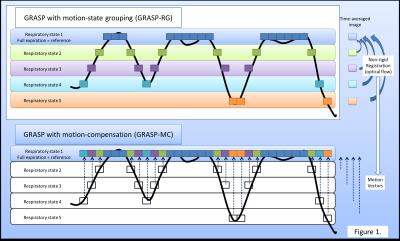
A method to combine information of respiratory states with a non-rigid motion model is proposed to reconstruct motion-compensated 4D images of dynamic-contrast enhanced MRI with high temporal resolution. Abdominal DCE-GRASP MRI was reconstructed by grouping 4 consecutive spokes as one dynamic frame (0.6sec/frame). Respiratory motion was obtained from central k-space data (as in XD-GRASP). A temporal TV constraint was applied separately for each respiratory state. By using an optical-flow algorithm, four sets of motion vectors were obtained. Motion-compensated GRASP reconstruction was performed by including the motion vectors and showed improved image quality and reduced motion blurring.
Background
Golden-Angle Radial Sparse Parallel (GRASP) MRI enables free-breathing continuous data acquisition and flexible reconstruction along the time dimension, which is particularly useful for dynamic contrast enhanced (DCE) MRI of the upper abdomen1,2. However, since GRASP MRI is performed under free breathing, the effect of the respiratory motion is still present in the reconstructed images. Extra-dimensional GRASP (XD-GRASP) is an extension of GRASP, which deals with this problem by sorting the dynamic data into different motion states using the self-navigation properties of radial imaging3. Because the image contrast changes in DCE-MRI scans, the sorting into motion states is usually done within discrete time windows (as it is not possible to mix, e.g., pre- and post-contrast data). This limits the temporal resolution achievable with XD-GRASP, which is therefore not sufficient for pharmacokinetic analysis. Previous studies have also shown that reconstruction from radial k-space data can include a non-rigid motion model in the low rank plus sparse (L + S) reconstruction4, but information of motion states which could be obtained from the data was not used in this technique. Here, we propose a method to combine information of respiratory states with a non-rigid motion model to reconstruct motion-compensated 4D images with high temporal resolution.
Purpose
The purpose of this study was to compare proposed method (GRASP with motion compensation; GRASP-MC) with GRASP with respiratory-state grouping (GRASP-RG) as well as GRASP.Materials and Methods
Dynamic abdominal images were obtained at 1.5 T (Siemens MAGNETOM Avanto, Erlangen, Germany) with a 12-element body array coil using the GRASP sequence (3D stack-of-star radial FLASH) with following parameters: TR/TE = 4.27/1.88 msec, flip angle = 12o, TA = 337 sec, voxel size =1.5 x 1.5 x 3 mm. Prior to the reconstruction, readout oversampling was removed in k-space, reducing the samples from 512 to 256. Only the initial 600 spokes were used to lower the computational burden in this experimental setup. Images were reconstructed by grouping 4 consecutive spokes into one dynamic frame. Temporal resolution was approximately 0.6sec/frame. Projection profiles from the entire volume for each angle were obtained from the central k-space data, and the data was processed by PCA to extract respiratory motion, as in XD-GRASP3. Unlike in XD-GRASP, a TV constraint is not applied along the respiratory-state dimension. Instead, the following cost function is minimized:
$$E(x)=||Ax-y||_2^2+\left\{\sum_{r=1}^R\lambda\cdot{}TV_t(x_{k(r)})\right\},(1)$$
where r is the respiratory state (with maximum number of respiratory states R=5), k(r) represents the index for the image frames (3D image volumes) at the respiratory state r. By applying the TV constraint separately for each respiratory state, we aimed to minimize the effect of respiratory motion in the reconstruction process. We call this reconstruction as GRASP-RG. As second step, the images were averaged over time separately for each respiratory-motion state, followed by nonrigid image registration by using an optical-flow algorithm (Fig. 1). The respiratory state with full expiration was used as a reference. Thus, a total of four sets of motion vectors were obtained. Using these motion vectors, and using the results of GRASP-RG as initial images, the final reconstruction (GRASP-MC) was then performed by minimizing the following cost function;
$$E(x)=||Ax-y||_2^2+\lambda\cdot{}TV_t(M_r(x_{k(r)})),(2)$$
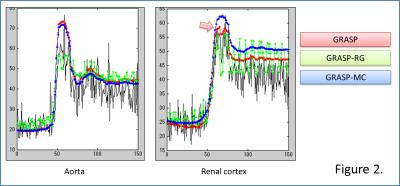
where Mr are the motion vectors for respiratory state r. Image analysis was performed as follows: at a representative axial slice of the 4D volume, ROIs were drawn for the aorta and renal cortex. Temporal changes in signal intensity (SI) for each ROI were compared among GRASP-MC, GRASP-RG, and GRASP. Images were reconstructed with an in-house developed Matlab script based on the FISTA algorithm for CS5. λ was empirically determined as 0.5 for this experiment. Reconstructions used 50 iterations for all stages of reconstruction. Images were also evaluated qualitatively by a board-certified radiologist.
Results
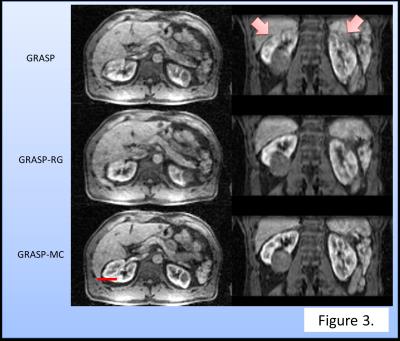
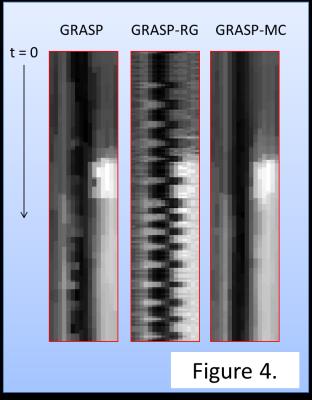
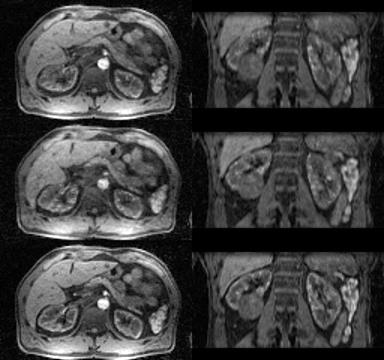
Temporal changes in SI for each reconstruction were summarized in Fig. 2. GRASP-RG suffered from signal variation due to motion. GRASP was inferior in acquiring the signal peak of the renal cortex. Qualitatively, GRASP showed blurring at the edge of the kidney (Fig. 3). GRASP-MC (proposed method) showed sharper images than GRASP-RG and GRASP (Fig. 3). Time-profile curves measured at the edge of the kidney are shown in Fig. 4. The reconstruction using GRASP-MC took approximately 8 minutes for one iteration.Discussion
Since respiratory motion is compensated in high temporal resolution DCE-MRI, the proposed technique is well suited for ROI- or voxel-by-voxel based pharmacokinetic analysis of biomarkers such as ktrans, kep and ve.Conclusion
GRASP-MC showed better image quality and smaller motion blurring than existing GRASP variants. Future work should include computational speed-up, as well as frame-wise image registration.Acknowledgements
This work is supported in part by the Grant-in-Aid for Scientific Research on Innovative Areas “Initiative for High-Dimensional Data-Driven Science through Deepening of Sparse Modeling” of The Ministry of Education, Culture, Sports, Science and Technology, Japan.(MEXT grant number 25120002).References
[1] Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI.Magn Reson Med. 2014;72(3):707-717.
[2] Chandarana H, Feng L, Block KT, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling.Invest Radiol. 2013;48(1):10-16.
[3] Feng L, Axel L, Chandarana H, et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing.Magn Reson Med. 2016;75(2):775-788.
[4] Otazo R, Koesters T, Candes E, et al. Motion-guided low-rank plus sparse (L+S) reconstruction for free-breathing dynamic MRI Proceedings of the 22nd Annual Meeting of the ISMRM, Milan, Italy; 2014. #0742
[5] Beck A, Teboulle M, A fast iterative shrinkage-thresholding algorithm for linear inverse problems SIAM Journal on Imaging Sciences 2009;2(1):183-202.
Figures