1990
A biodegradable macromolecular MRI contrast agent for enhanced liver metastasis1Radiology, Sir Run Run Shaw Hospital, Zhejiang, People's Republic of China, 2Chemical and Biological Engineering, Zhejiang University, Zhejiang, People's Republic of China, 3Life Science, GE healthcare, Shanghai, People's Republic of China
Synopsis
Tumor metastasis accounts for the related mortality, and precise diagnostic imaging of distant metastasis plays a significant role in clinical administration and treatment plan. Enhanced MRI with small-molecule contrast agent (CA) is a favorite imaging modality. However, small-molecule CA has some unsatisfied flaws such as short blood circulation time, low relaxivity and non-specificity. In this study, we synthesized a macromolecular CA with high relaxivity and long blood circulation time, and assessed early liver metastasis with enhanced MRI using this macromolecular CA.
Purpose
To
develop a biodegradable dendrimer-based macromolecular CA to improve the detection
sensitivity of MRI in liver metastasis.Introduction
Metastasis is one of the main reasons for cancer-associated mortality1 and needs accurate diagnosis in time. Magnetic Resonance imaging (MRI), with small-molecule contrast agents (e.g. DTPA-Gd and DOTA-Gd) is one of the most important imaging modalities2. However, these small-molecule agents have some limitations resulting in low efficient detection in small tumors and early metastasis. Macro-molecular CAs, which are polymers conjugated with Gd-chelates, have higher relaxivity and longer blood circulation time. In addition, many dendritic CAs(DCAs) are currently based on non-biodegradable dendrimers including polyamidoamine (PAMAM) and polypropyleneimine (PPI) that cannot be excreted effectively from the body and be metabolized by cells releasing toxic Gd3+ ions3. In our present study, we developed a novel biodegradable dendrimer with high relaxivity and prolonged blood circulation time to detect liver metastasis.Method
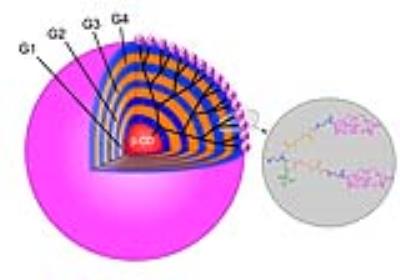
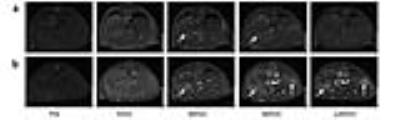
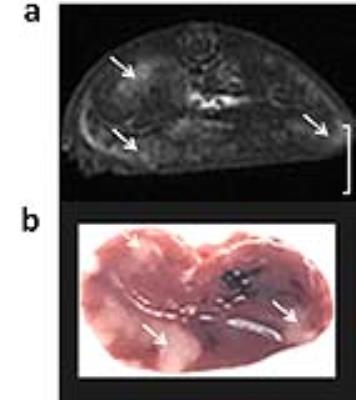
A biodegradable macromolecular CA, G4-MOP-DTPA-Gd, was synthesized with β-CD core (β-cyclodextrin) and modified with DTPA-Gd. The size of CA, molecular weight and relaxivity, were measured, and in-vitro MRI and degradation experiments were performed. A mice model of liver metastasis was constructed by injecting 4T1-Luc breast cancer cells (1×106 in 50μL PBS) into the parenchyma of spleen of 6-8 week-old female BALB/c mice. Images were acquired with a fat suppression T1-weighted FSE using a 3T MRI system (Discovery 750w 3T, GE Healthcare). MRI studies were performed at pre-injection and 5, 30, 60, 120 min post injection of CA. Contrast to noise ratio (CNR) and tumor/liver enhancement ratio were calculated and plotted with time. The mice were killed immediately after serial examinations, fixed in formaldehyde and then sliced in the same planes as the MR sectional images.Results and discussion
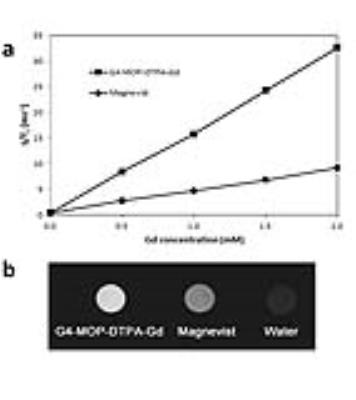
A novel MRI biodegradable dendritic CA, G4-MOP-DTPA-Gd, with a molecular weight of 337KDa was successfully synthesized (figure 1). G4-MOP-DTPA-Gd showed a longer half-life in blood circulation and higher longitudinal relaxivity (r1, 15.7 mM-1s-1) (figure 2). The CNR of the intrahepatic metastasis using G4-MOP-DTPA-Gd had a significant increase, and was much higher than that of Magnevist (figure 3). The images obtained 60 min and 120min after injection showed a gradual increase in signal intensity in the metastatic tumor at the periphery of the liver(figure 5). The MR images enhanced with G4-MOP-DTPA-Gd at 30min post injection correlated well to the corresponding histological section(figure 5).Conclusion
G4-MOP-DTPA-Gd, a positive CA of liver, had higher CNR in tumor lesions, allowing MR imaging with earlier detection of liver metastasis. In addition, the post-injection images with this DCA were more useful to detect smaller tumors at the periphery of the liver lobe and directly to separate these from hepatic vessels. A biodegradable DCA with less influence of normal hepatic function may help to clear away some obstacles from basic to clinical research.Acknowledgements
No acknowledgement found.References
1. Weis C, Blank F, West A, et al. Labeling of Cancer Cells with Magnetic Nanoparticles for Magnetic Resonance Imaging. Magn. Reson. Med. 2014; 71:1895-1905.
2. Bae MS, Shin SU, Ryu HS, et al. Pretreatment MR imaging features of triple-negative breast cancer: association with response to neoadjuvant chemotherapy and recurrence-free survival. Radiology 2016; 281, 392-400.
3. Tang J, Sheng Y, Hu H, Shen Y. Macromolecular MRI contrast agents: Structures, properties and applications. Progress in Polymer Science 2013; 38, 462-502.
Figures