1989
Towards an Early Detection of Prostate Cancer using Zinc-Sensitive iCEST MRI1The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University, Baltimore, MD, United States
Synopsis
As compared to normal prostate tissue, prostate cancer cells exhibit a dramatic reduction in zinc content. We have used 19F-based ion-CEST (iCEST) MRI to sense differential Zn2+ levels between normal and malignant prostate cell lines. We were able to observe clear differences in zinc-induced iCEST signal between normal cells and cancer cells, which was validated by microscopy using a zinc-sensitive fluorescent dye. Hence, iCEST MRI may have potential as a new means to non-invasively detect early prostate malignant transformation.
Target Audience: Researchers, radiologists and other clinicians interested in prostate cancer imaging, 19F MRI, and CEST.
Purpose: To develop a novel zinc-sensitive CEST MRI method for early detection of prostate cancer.
Introduction: The healthy prostate contains the highest concentrations of mobile zinc of all soft tissues in the body. These levels decreasedramatically during the development of prostate cancer. Prostate cancer is the only known disease of the prostate that displays such a substantial decrease in tissue zinc content and neither prostatitis nor benign prostatic hyperplasia are associated with this phenotype. Therefore, non-invasive detection of prostate zinc content may be applied for an early diagnosis of malignant transformation1,2. Indeed, several MRI methods have been used to probe the cellular Zn2+ content, based on the use of 1H MRI with paramagnetic probes2,3. A new approach is to use 19F MRI with fluorinated probes, termed ion CEST or iCEST, that is capable to detect sub-millimolar Zn2+ concentrations4,5. Here, we assessed whether or not iCEST MRI can be used to differentiate between normal and malignant prostate cells.
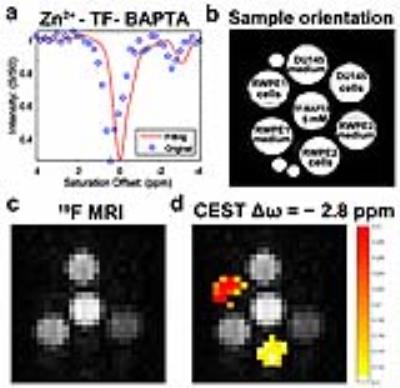
Methods: Cell lines: Three commercial human cell-lines (ATCC) were used: normal (RWPE1 and RWPE2) and malignant (DU145) prostate cells. Fluorescence imaging: Cells were incubated with or without ZnCl2 (100 μM), and then treated with the zinc-sensitive fluorescent dye N-(6-methoxy-8-quinolyl)-p-carboxybenzoyl-sulphonamide (TFLZn) potassium salt, using propidium iodide (PI) as counterstain. Cell cytotoxicity assay: Cytotoxicity was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 19F and iCEST MRI: Cells were incubated with 50 μM ZnCl2 and then treated with 1,2-bis(2-amino-5,6-difluorophenoxy)ethane-N,N,N',N'-tetraacetic acid (TF-BAPTA). After centrifugation, the supernatant and cells were collected in 5 mm NMR tubes. MRI experiments were performed on a vertical 17.6 T scanner (Bruker Avance system). For 19F MRI, a RARE sequence was used with the following parameters: Repetition time (TR)/echo time (TE)=500/5.79 ms; RARE factor=16; 20 mm slice thickness; FOV=2.0×2.0 cm; matrix size=32×32; resolution=0.78×0.78 mm, and one average (NA=15,000). For 19F iCEST MRI, the center frequency (O1) was set at the frequency of the 19F atom at the 6 position (0.0 ppm) of TF-BAPTA. A modified RARE sequence (TR/TE=3,053/2.87 ms, RARE factor=16, 20 mm slice, FOV=2×2 cm, matrix size=32×32, resolution=0.78×0.78 mm, NA=270 and a saturation pulse B1= 2.4 µT/ 3 s) was used to acquire the 19F iCEST data.
Results: The occurrence of different Zn2+ levels between normal and malignant prostate could be validated using fluorescence microscopy, demonstrating an intracellular distribution of TFLZn at high magnification (Fig. 1a,b). No cytotoxicity of the iCEST probe could be observed for 10 mM or lower TF-BAPTA (Fig. 1c). Five mM TF-BAPTA was therefore chosen for further studies. Both the supernatant (incubation medium) and cell pellets were imaged with 19F and iCEST MRI (Fig. 2). Only the supernatant and the TF-BAPTA itself were visible on 19F MRI, but not the cell-pellets (Fig. 2c). Fig. 2d shows the iCEST image acquired at the frequency of Zn2+-bound TF-BAPTA (-2.8 ppm from 19F), overlayed on the 19F image. An iCEST signal from the Zn2+-bound TF-BAPTA can be clearly observed in the normal prostate cell lines RWPE1 and RWPE2, but not in malignant DU145 cells. Note that the iCEST has a lower Zn2+ signal for RWPE2 cells compared to RWPE1, as was the case for fluorescence microscopy (Fig. 1).
Discussion and Conclusion: Both fluorescence imaging and iCEST MRI show clearly detectable differences in zinc concentration between normal and malignant prostate cells. Hence, iCEST MRI may have potential as a new means to non-invasively detect early prostate malignant transformation.
Acknowledgements
We thank the Dr. Zhiliang Wei and Dr. Guanshu Liu for help with CEST data processing. This project was supported by R03 EB018882 and the Pearl and Yueh-Heng Yang Foundation.References
1. Ghosh SK, Kim P, Zhang XA, Yun SH, Moore A, Lippard SJ, Medarova Z. A novel imaging approach for early detection of prostate cancer based on endogenous zinc sensing. Cancer Res. 2010;70(15):6119-6127.
2. Clavijo Jordan MV, Lo ST, Chen S, Preihs C, Chirayil S, Zhang S, Kapur P, Li WH, De Leon-Rodriguez LM, Lubag AJ, Rofsky NM, Sherry AD. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc. Natl. Acad. Sci. U. S. A. 2016;113(37):E5464-5471.
3. Lubag AJ, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Noninvasive MRI of beta-cell function using a Zn2+-responsive contrast agent. Proc. Natl. Acad. Sci. U. S. A. 2011;108(45):18400-18405.
4. Bar-Shir A, Gilad AA, Chan KW, Liu G, van Zijl PC, Bulte JW, McMahon MT. Metal ion sensing using ion chemical exchange saturation transfer 19F magnetic resonance imaging. J. Am. Chem. Soc. 2013;135(33):12164-12167.
5. Bar-Shir A, Yadav NN, Gilad AA, van Zijl PC, McMahon MT, Bulte JW. Single 19F probe for simultaneous detection of multiple metal ions using miCEST MRI. J. Am. Chem. Soc. 2015;137(1):78-81.
Figures