1983
Simultaneous Acquisition of Multiple Z-spectra using Sinc-Modulated RF Pulse Trains in Gradient Encoded CEST MRIHirohiko Imai1, Kiyotaka Miyake2, and Tetsuya Matsuda2
1Center for the Promotion of Interdisciplinary Education and Research, Kyoto University, Kyoto, Japan, 2Department of Systems Science, Graduate School of Informatics, Kyoto University, Kyoto, Japan
Synopsis
We propose a use of sinc-modulated RF pulse train instead of the conventional continuous wave RF irradiation under the presence of constant gradient as a saturation scheme in chemical exchange saturation transfer (CEST) MRI aiming at a simultaneous acquisition of multiple Z-spectra. The proposed method was applied for a glutamic acid solution in water. The multiple Z-spectra could be observed along the gradient encoding direction by repetitive application of the saturation scheme. Thus, the present study shows the potential of the proposed methodology for accelerating the CEST MRI.
INTRODUCTION
Chemical exchange saturation transfer (CEST) MRI can sense the presence of low-concentration solutes containing chemically exchangeable protons.1 To quantify the CEST effect, a series of images or spectra collected with a different saturation frequency offset is required for generating Z-spectrum.1 To accelerate Z-spectrum acquisition, ultrafast Z-spectroscopy (UFZ)2-5 or ultrafast CEST imaging (UCI)6,7 has been developed, in which spectral information is encoded along a spatial dimension using a magnetic field gradient during an irradiation of constant wave (CW) RF pulse for saturation. Although this ultrafast approach requires just two scans for obtaining Z-spectrum, only a single Z-spectrum can be acquired for the gradient encoding direction, i.e., no spatial information about the target molecules could not be obtained along this direction. In the present study, we propose a use of an RF pulse train instead of the conventional CW for saturation in the UFZ methodology aiming at the simultaneous acquisition of multiple Z-spectra along the gradient encoding direction.METHODS
The pulse sequence used for this study is illustrated in Fig.1a. A train of rectangular RF pulses in conjunction with a constant gradient was applied for saturation transfer (ST) preparation followed by an image acquisition module. The RF pulse train was modulated by a three-lobe sinc function8 and its power was adjusted to be a total flip angle of π/2. The frequency of the RF pulses was set at a resonance of water proton without a gradient field. The saturation encoding gradient (Gsat=1.41G/cm) was applied along the readout direction followed by a crusher gradient. The ST block was repeated N times to enhance the CEST effect. Figure 1b shows the excitation profile for N=1. For the water protons, the direct water saturation with a 0.2mm width occurs every 5mm separation with the position at 0mm as a center (Fig.1b,black). Assuming a presence of solute exchangeable protons having a resonant frequency difference of Δω=900Hz with respect to water (chemical shift difference of 3ppm at 7T), the excitation profile for the solute protons shifts a distance d=Δω/γGsat,, which is 1.5mm in the present study (Fig.1b,red). Therefore the solute protons located at these positions are saturated and exchange with the water protons, generating a multiple Z-spectra. In the experiments, glutamic acid solution in water (50mM) was used where the exchangeable amide protons at 3ppm were targeted. 3ml of the solution was sealed in a cylindrical vessel (20mm i.d.). MR scans were performed on a Bruker 7T MR system (BioSpec 70/20 USR) using a quadrature transmit-receive volume coil. The dependence of the CEST effect on N ranging from 1 to 600 was investigated, which corresponds to the ST preparation period (tsat) of 19.3ms-11.6s. For image acquisition, RARE sequence was used following ST preparation with parameters: TR=tsat+2.5s, effective TE=16.6ms, RARE factor=8, a centric ordered phase encoding, slice thickness=2mm, matrix size=500×256 and FOV=50×25.6mm2. Depending on the tsat, the acquisition time for each image took 1.3-7.5min.RESULTS AND DISCUSSION
Figure 2a shows acquired images with N=1 and N=300. For the N=300, beside the wide dark bands, which corresponds to the direct water saturation, lines are seen as slightly reduced signal intensity (indicated by red arrow) as a result of the chemical exchange saturation transfer. This CEST effect cannot be found in the image for N=1, which is due to the insufficient accumulation of saturated protons in water pool. An intensity profile along the encoding gradient direction extracted from normalized image of N=300 (Fig.2c) shows that multiple Z-spectra can be obtained with clear CEST effects at positions approximately 1.5mm apart from positions of water direct saturation. Figure 3a shows N dependence of normalized images. Although the sinc-modulated RF pulse train yields sharper-edged excitation profile, the width of direct water saturation broadened as a result of the repetitive application of the pulse trains. For increasing the number of Z-spectra within unit length, i.e., achieving high spatial resolution Z-spectroscopy, the suppression of this broadening would be required. By using the Z-spectra extracted from these images, a magnetization transfer ratio asymmetry (MTRasym) analysis was performed.1 Figure 3b shows the N dependence of the MTR asymmetry curves. The values at 3ppm were plotted as a function of N as well as the corresponding tsat (Fig.3c). The value was reached nearly plateau around N=300(tsat=6s).CONCLUSION
We have demonstrated that the multiple Z-spectra can be obtained simultaneously by using a sinc-modulated RF pulse trains in UFZ methodology. While the proposed method requires further optimization, this study shows its potential for accelerating CEST MRI.Acknowledgements
This work is partly supported by JSPS KAKENHI Grant Number JP15K01283.References
1. van Zijl PCM, et al. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t. Magn. Reson. Med. 2011;65:927-948. 2. Xu X, et al. Ultrafast scanning of exchangeable sites by NMR spectroscopy. Angew. Chem. Int. Ed. 2013;52:8281-8284. 3. Döpfert J, et al. Slice-selective gradient-encoded CEST spectroscopy for monitoring dynamic parameters and high-throughput sample characterization. J. Magn. Reson. 2013;237:34-39. 4. Liu Z, et al. UCEPR: Ultrafast localized CEST-spectroscopy with PRESS in phantom and in vivo. Magn. Reson. Med. 2016;75:1875-1885. 5. Wilson NE, et al. Localized, gradient-reversed ultrafast Z-spectroscopy in vivo at 7T. Magn. Reson. Med. 2016;76:1039-1046. 6. Döpfert J, et al. Ultrafast CEST imaging. J. Magn. Reson. 2014;243:47-53. 7. Xu X, et al. Screening CEST contrast agents using ultrafast CEST imaging. J. Magn. Reson. 2016;265:224-229. 8. Wu EX, et al. MRI cardiac tagging using a sinc-modulated RF pulse train. Magn. Reson. Med. 2002;48:389-393.Figures
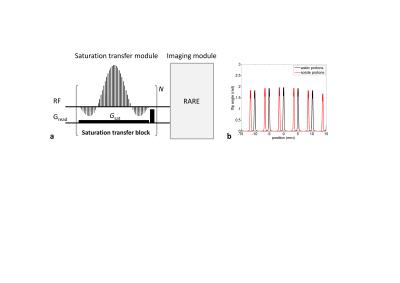
Fig.1. a: Pulse sequence used in the present study.
The parameters for the saturation transfer
preparation were: duration of individual RF pulses=33.3us, duration
of the sinc-modulated RF pulse train=16.66ms(main lobe=8.33ms),
spacing
of consecutive RF pulses=333us, the number of RF
pulses=50, the
maximum B1=15.6uT, saturation encoding gradient Gsat=1.41G/cm,
and duration of the saturation preparation block=19.3ms. b: Excitation profile of the saturation transfer preparation
with N=1 for water protons (black)
and for the solute protons with a chemical shift difference of 3ppm with
respect to the water resonance (red). Gradient center is assigned to the
position at 0mm.
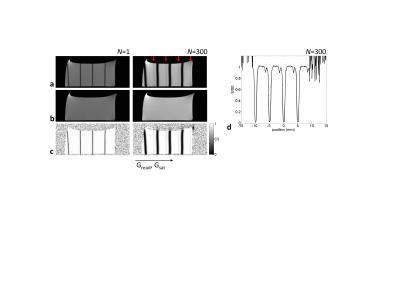
Fig.2. a,b: Acquired images in 50mM of
glutamic acid solution with (a) and without (b) saturation, which produce
normalized images (lower) for obtaining Z-spectra, for N=1 (left) and N=300
(right). Red arrows indicate the signal reduction caused by chemical exchange
saturation transfer. c: Normalized
image intensity profile from image with N=300.
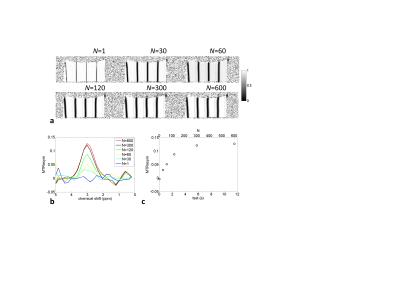
Fig.3. a: Normalized images for varied N.
b: N dependence of MTRasym
curves. The water frequency assigned to 0ppm. c: Plot of the MTRasym values at 3ppm in b as a function of N as well as tsat.