1982
The origins of CEST contrast in ischemic tissue: effects of hypotonic stress on the nervous system of Aplysia californica1NeuroSpin, Commisariat à l'Energie Atomique et aux Energies Alternatives, Gif-sur-Yvette, France
Synopsis
Recent CEST MRI methods such as Amide Proton Transfer (APT) imaging allow the detection of brain tumors and stroke by generating novel image contrasts which depend on the chemical exchange. In this paper, we are studying the effects of ischemia on the CEST signal at a tissue level using the nervous system of Aplysia californica, a widespread model in neuroscience. A significant change in the Z spectrum was observed at 2.5 ppm after hypotonic shock in the abdominal ganglion and was quantified as a +2.88% MTR increase. Cell swelling, which is a known phenomenon in ischemic tissue, could potentially cause such effect.
Purpose
Chemical Exchange Saturation Transfer (CEST) imaging is receiving growing interest in the MRI community.1,2 Recent CEST MRI methods such as Amide Proton Transfer (APT) imaging3 allow the detection of brain tumors4 and stroke5 by generating novel image contrasts which depend on the chemical exchange between water and the amide protons of mobile proteins and peptides. While the exact origin of the CEST contrast in ischemic tissue is still in debate, a well-accepted hypothesis is that the amide exchange rates are highly sensitive to tissue acidosis.6 In this paper, we are studying the effects of ischemia on the CEST signal at a tissue level using the nervous system of Aplysia californica. The Aplysia is a widespread model in neuroscience and has previously been used for single cell MR diffusion measurements.7 Localized CEST spectra were acquired at 17.2 T from ganglia tissue before and after hypotonic shock. The observed changes in the CEST spectrum were quantified and discussed.Method
Animal preparation. Aplysia californica were anesthetized by injection of MgCl2 solution. The abdominal ganglia were resected and inserted into a 2 mm-diameter glass capillary filled with Artifical Sea Water (ASW). Two imaging sessions were carried out: one before and one after a 30 minute stress period consisting in exposure to a 33% hypotonic ASW solution. A total of 3 ganglia were scanned.
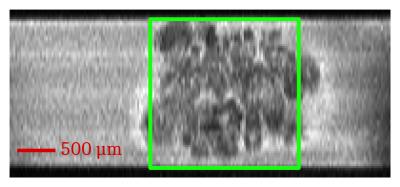
MRI acquisitions. All experiments were performed using a 17.2 T magnet (Bruker BioSpin) using home-built micro-coils as RF transceivers. 3D spin-echo rapid acquisition with relaxation enhancement (3D RARE) images (TE/TR=10/1500 ms) were acquired from a 5.0x2.2-mm field of view leading to a 25x68-µm in-plane resolution (Fig. 1). Localized shimming using FASTMAP resulted in, on average, 20 Hz water linewidths. Localized CEST spectra were acquired using a custom PREST (Point REsolved Saturation Transfer) sequence which consisted in a CEST preparation followed by a PRESS detection. The CEST preparation achieved a B1 of 3.5 µT using a train of 40 50-ms rectangular pulses with a frequency offset variation between –10 and +10 ppm relative to the water resonance (44 increments). The signal was acquired from a 8 mm3 PRESS voxel (TE/TR=10/2500ms).
Data processing. Z spectra were processed with a customized MATLAB code. Magnetization Transfer Ratio (MTR) spectra were generated using the following equation: $$MTR(\Delta w)=\frac{S(-\Delta w)-S(+\Delta w)}{S_{ref}}$$ where $$$\Delta w$$$ is the frequency difference with water and $$$S_{ref}$$$ is the reference signal acquired when the saturation frequency is off-resonance (-10 ppm in this study).
Results
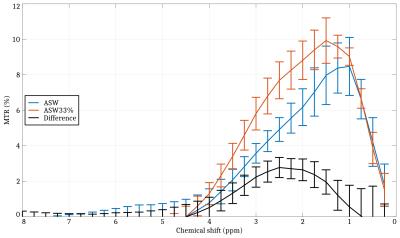
Fig. 1 shows the anatomical RARE image acquired in order to place the PREST voxel. Fig. 2 shows the MTR spectra obtained before and after hypotonic shock, averaged over 3 different ganglia. A significant increase in MTR of +2.88% is observed at 2.5 ppm.Discussion & Conclusion
A significant change in the Z spectrum was observed after hypotonic shock in the Aplysia californica abdominal ganglion and was quantified as a +2.88% MTR increase for a chemical shift centered at 2.5 ppm. Most studies using CEST MRI methodologies to detect brain strokes are aiming at amide protons (3.5 ppm) because their exchange rates increase with pH. McVicar recently showed that amine protons (2.75 ppm) have an opposite dependence with pH, allowing absolute pH mapping by measuring the ratio amine/amide.8 While the ASW and the 33% hypotonic ASW solutions were carefully pH-calibrated to 7.0 in our experiments, we cannot exclude the possibility of a local pH drop in the ganglion during and after the hypotonic shock. A possible explanation for the results we obtained could be that the hypotonic shock induces micro-structural cellular changes allowing an increased bounding between water protons and mobile proteins carrying amine protons. The latter could likely cause an increase of chemical exchange effects at specific chemical shifts or it could induce higher CEST rates. Cell swelling, which is a known phenomenon in ischemic tissue, could potentially lead to such a scenario. If such hypothesis is validated, it could open new ways of studying cell swelling during neuronal activation.9 To conclude, this paper presents an attempt at understanding the mechanisms underlying ischemia using CEST measurements at a tissue level on the Aplysia californica model.Acknowledgements
This research was supported by the CEA Enhanced Eurotalents program, the Louis-Jeantet Foundation and a public grant overseen by the French National Research Agency (ANR) under the project name “ANImE” (reference: ANR-13-BSV5-0014-01).References
1. Zaiss M and Bachert P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and methods. Phys Med Biol. 2013;58:R221-R269.
2. Kogan F, Hariharan H and Reddy R. Chemical Exchange Saturation Transfer (CEST) Imaging: Description of Technique and Potential Clinical Applications. Curr Radiol Rep. 2013;1:102-114.
3. Zhou J. Amide proton transfer imaging of the human brain. Methods Mol Biol. 2011;711:227-237.
4. Zhou, J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu D-X, Ford E, Tyler B, Blakeley J, Laterra J and van Zijl PCM. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med. 2011;17:130-134.
5. Sun PZ, Wang Y and Lu J. Sensitivity-enhanced chemical exchange saturation transfer (CEST) MRI with least squares optimization of Carr Purcell Meiboom Gill multi-echo echo planar imaging. Contrast Media Mol Imaging. 2014;9:177-181
6. Jin T, Wang P, Zong X and Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med 2013;69:760-770.
7. Jelescu IO, Ciobanu L, Geffroy F, Marquet P and Le Bihan D. Effects of hypotonic stress and ouabain on the apparent diffusion coefficient of water at cellular and tissue levels in Aplysia. NMR Biomed. 2014;27:280-290.
8. McVicar N, Li AX, Gonçalves DF, Bellyou M, Meakin SO, Prado MA and Bartha R. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab. 2014;34:690-698.
9. Le Bihan D, Urayama SI, Aso T, Hanakawa T and Fukuyama H. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad Sci U S A. 2006;103:8263-8268.
Figures