1981
Rapid 3D CEST using volumetric reduced field of view imagingJianbo Shao1, Bing Wu2, and Hui Lin3
1Wuhan Children Hospital, Wuhan, People's Republic of China, 2GE healthcare MR Research China, Beijing, People's Republic of China, 3GE healthcare MR Research China
Synopsis
A reduced field of view CUBE acquisition was proposed for CEST imaging. It is advantageous in situations where the imaging volume may be constrained to a region of the whole volume. Clinical feasible acquisition (10s per volume) was achieved at 2mm isotropic resolution.
Purpose
CEST (Chemical exchange saturation transfer) imaging is usually limited to single or several slices, as acquisition at multiple spectral offsets as well as sufficiently long repetition time are needed. 3D acquisition is desirable for volumetric measurements and also improved SNR level. In the past, several approaches of 3D CEST acquisition have been proposed: 3D GRASE [1], 3D EPI readout [2], 3D stack of spiral [3]. However these approaches is either poor on temporal resolution or poor on spatial resolution. A prior knowledge may be explored is the fact that the imaging volume of interest is often known, either via previous scans that may reveal the location of the lesions or the anatomical structure is relatively small such as the prostate. If the imaging volume may be reduced to be a portion of the entire anatomy, the acquisition time may be proportionally reduced. In this work, we investigate the feasibility of using reduced field of view 3D FSE (CUBE) acquisition in CEST imaging to achieve a better comprise between spatial and temporal resolution, when the scan region of interest may be known a priori.Method and experiements
The pulse sequence of reduced FOV CUBE with outer volume suppression (OVS) [4] based CEST is shown in Fig.1. A non-spatial selective Fermi windowed RF with crusher gradients is used as CEST saturation, followed by the very selective suppression (VSS) RF with quadratic phase modulate for suppressing the region outside the reduced FOV. Then fat suppression is played, finally a fast spin echo chain with a fast recovery strategy is used for readout. A duration of the train of the VSS pulses was about 60ms. APT imaging using the proposed method as well as conventional 2D EPI and 2D SSFSE were performed. The details of scan parameters of different CEST imaging are summarized in Table 1. In the current setup of rFOV CUBE acquisition, coverage of 25.6x6.4x6.4cm may be completed within 10s at a spatial resolution of isotropic 2mm resolution. Three patients with clinically confirmed brain tumors were enrolled in this study, consent forms were obtained. All the participants underwent conventional MRI scans including T1, T2 and contrast enhanced T1 FLAIR on a 3.0T whole body scanner (MR750, GE, USA). The imaging volume for rFOV was defined based on T1 scans, encompassing the entire lesions with sufficient spatial margins.Results
The anatomical as well as APT maps obtained from different CEST imaging methods of a patient with high grade glioma are shown in Fig.2. It can be seen that similar asymmetric level of MTR was obtained using the three different methods, and the that of rFOV CUBE tended to be slightly lower. This might be due to the extra OVS module after the CEST saturation. To study the volumetric measurement, the histogram of calculated asymmetrical MTR over the tumor lesion avoiding the fluid region is shown in Fig.3. The contrast enhanced T1 FLAIR was used to define the region of interest. It is seen that the overall level of APT effects fell within expected range at 3.0T.Discussion and conclusion
In this work, a reduced field of view CUBE acquisition was proposed for CEST imaging. It is advantageous in situations where the imaging volume may be constrained to a region of the whole volume. Clinical feasible acquisition (10s per volume) was achieved at 2mm isotropic resolution. The drawback of the use of outer volume suppression is the extra delay after the RF preparation which may reduce the CEST effects. In this work, the histogram based analysis of the brain tumor volume was also attempted. The feasibility of low distortion volumetric CEST imaging may allow more advanced quantitative analysis such as radiomics to be used.Acknowledgements
No acknowledgement found.References
[1] H. Zhu, et al. MRM. 2010. [2] G. Jones, et al. MRM 2011. [3] B. Wu, et al. ISMRM 2016. [4] M. Han, et al. JMRI 2015.
Figures

Table 1 Scan
parameters used for different strategies of CEST imaging

Figure
1 pulse sequence diagram of the proposed reduced field of view CEST imaging.
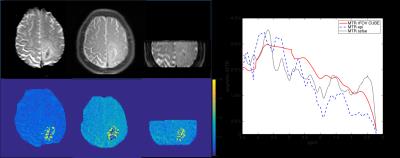
Figure
2 An axial APT map of a high grade glioma patient at 3.5ppm. Similar levels of
APT effects were observed using different acquisitions strategies, whereas the
measured assymetrical MTR was lower in reduced field of view CEST.
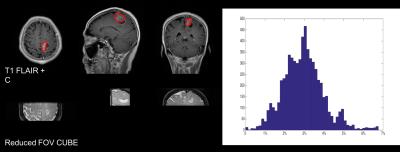
Figure
3 histogram analysis of the tumor leision, as defined by contrast enhanced T1
flair.