1972
isoAPTR* - a novel method to measure tumour pHi using CEST MRI1Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom, 2Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford
Synopsis
Quantitative CEST MRI studies have so far been hindered by the fact that variations in multiple factors produce identical CEST effect changes. This remains the case when CEST MRI data are analysed using metrics that control for the contaminating effects of T1 and T2, such as APTR*. In this work we introduce isoAPTR*, a novel methodology which, in combination with independent measurement of labile proton concentration, can estimate the change in intracellular pH between two APTR* measurements. We demonstrate the utility of this method by applying it to measure the intracellular pH of U87 glioma in rats.
Introduction
Quantitative CEST MRI studies have so far been hindered by the fact that changes in multiple factors can produce identical CEST effect changes. Recent work has demonstrated that the model-based quantification metric APTR* is optimally specific to changes in the exchanging proton pool compared to other CEST MRI analysis methods, eliminating the contributing effects of relaxation time changes1. However, converting APTR* measurements to a quantitative measurement of either of the remaining components, pH or labile proton concentration, is non-trivial since multiple combinations of changes in pH or labile proton concentration will generate the same observed change in APTR*. In this work, we present isoAPTR*, a novel methodology to analyse changes in APTR* and provide estimates of intracellular pH (pHi) changes in tumours as measured by CEST MRI.Methods
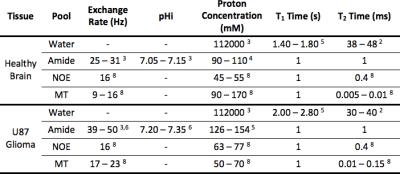
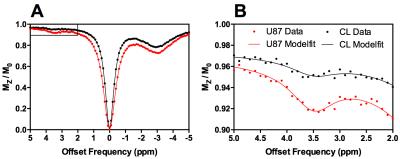
Z-spectra for healthy rat brain (n=200) and U87 glioma (n=200) were simulated using the parameters in Figure 1. Ranges of parameters were used, with each Z-spectrum simulated with a random combination of parameters, and Gaussian noise was added to reflect physiological variation. These Z-spectra were fit to the Bloch-McConnell equations using a Bayesian model-based algorithm2 and the APTR* metric used to quantify the CEST effect at 3.5ppm. The mean and 95% confidence interval (CI) of APTR* for healthy rat brain and U87 glioma was calculated from the 200 measurements, and compared using a two-tailed unpaired t-test.
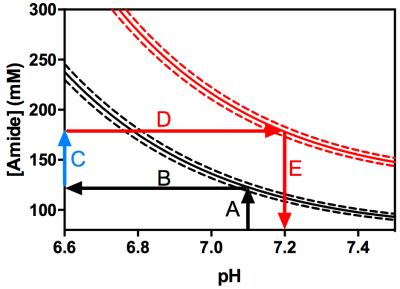
Over 500,000 theoretical APTR* values were calculated for a range of relative amide proton concentrations (0–4x10-3, corresponding to 0–376mM for healthy brain or 0–390mM for U87 glioma owing to different water content in the two tissues, n=501) and pH (5.00–7.65, n =1001). Lines of constant APTR* (‘isoAPTR* lines’) were plotted for the mean and 95% CI of APTR* as measured in healthy rat brain and U87 glioma.
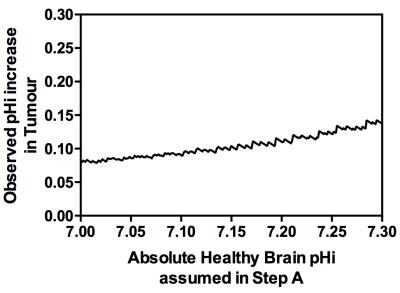
Quantitative estimates of either parameter (pH or amide proton concentration) can then be made assuming the other is known. A known healthy brain pHi was assumed to estimate the healthy rat brain amide proton concentration. Subsequently, using the ratio of 1.40 as previously measured from cytoplasmic protein concentrations in U87 glioma and healthy brain7 to infer an increase in amide proton concentration, estimates of the tumour pHi were made. Since the observed pHi increase in tumour depends on the initial estimate of the absolute healthy brain pHi, the isoAPTR* method was repeated with variable assumed healthy brain pHi to investigate the resilience of the isoAPTR* method to the initial estimate of healthy brain pHi.
Results
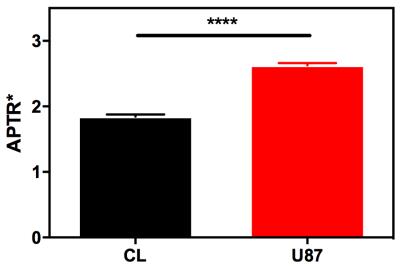
The Bayesian model-based algorithm fit the data for healthy rat brain and U87 glioma very well (Fig 1). The calculated mean and 95% CI of APTR* in healthy rat brain (1.82±0.06%) and U87 glioma (2.77±0.06%) were significantly different (Fig 2; unpaired t-test, P < 0.0001). The isoAPTR* method (Fig 3) measured a pHi increase in tumour compared to healthy brain tissue of 0.11±0.02 pH units over a wide range of assumed healthy brain pHi values (7.00–7.30, Fig 4).Discussion
Using the novel isoAPTR* method, an increase in pHi in the U87 glioma compared to healthy rat brain has been successfully measured from these simulated CEST MRI data. The isoAPTR* method serves as further demonstration of the specificity of APTR* to amide proton exchange effects, rather than contamination from T1 and T2 relaxation effects, in agreement with recent in vitro studies1.
The proposed isoAPTR* method relies on an independent (i.e. non-CEST MRI derived) measurement of protein concentration, which in this study has been taken from previous literature7. The healthy brain pHi must also be assumed to make tumour pHi estimates. A number of studies have established that the healthy brain pH is well-regulated and in the range 7.00 – 7.203,4. For healthy brain pHi in this range, the tumour pHi estimated by isoAPTR* is relatively insensitive to the initial estimate of healthy brain pHi.
While biochemical protein concentration estimates of brain tissue may not be available routinely in a clinical environment, the isoAPTR* method still provides a useful mechanism by which to study the origins of the CEST effect in preclinical settings.
Conclusion
isoAPTR* is a novel, quantitative CEST MRI analysis method that measures tumour pHi from CEST MRI data, quantified using the APTR* metric.Acknowledgements
This work was funded by Cancer Research UK (grant number C5255/A15935), the CRUK & EPSRC Cancer Imaging Centre in Oxford (grant number C5255/A16466). K.J.R. was funded by a Medical Research Council studentship (MC_ST_U13080) and supplementary award (MR/K501256/1)References
1. Ray, K. J. et al. Determination of an optimally sensitive and specific chemical exchange saturation transfer MRI quantification metric in relevant biological phantoms. NMR Biomed. 29, 1624–1633 (2016).
2. Chappell, M. A. et al. Quantitative Bayesian model-based analysis of amide proton transfer MRI. Magn. Reson. Med. 70, 556–567 (2013).
3. Zhou, J., Payen, J. F., Wilson, D. A., Traystman, R. J. & van Zijl, P. C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 9, 1085–1090 (2003).
4. Tee, Y. K. et al. Comparing different analysis methods for quantifying the MRI amide proton transfer (APT) effect in hyperacute stroke patients. NMR Biomed. 27, 1019–29 (2014).
5. Xu, J. et al. On the origins of chemical exchange saturation transfer (CEST) contrast in tumors at 9.4 T. NMR Biomed. 27, 406–16 (2014).
6. Mcintyre, A. et al. Disrupting Hypoxia-Induced Bicarbonate Transport Acidifies Tumor Cells and Suppresses Tumor Growth. Cancer Res. 76, OF1-OF12 (2016).
7. Yan, K. et al. Assessing Amide Proton Transfer (APT) MRI Contrast Origins in 9 L Gliosarcoma in the Rat Brain Using Proteomic Analysis. Mol. Imaging Biol. 17, 479–87 (2015).
8. Lee, D.-H. et al. Quantitative assessment of the effects of water proton concentration and water T 1 changes on amide proton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: The origin of the APT imaging signal in brain tumor. Magn. Reson. Med. (2016). doi:10.1002/mrm.26131
Figures