1931
Origin of Dipolar Effects - Achilles Tendon at 3T and 11.7T.1Radiology, Univ. of California, San Diego, San Diego, CA, United States
Synopsis
We examined the MR image appearance of human Achilles tendon as a function of orientation to the B0 field at 3 T and 11.7 T. Images were registered and displayed a remarkable similarity in the fine discernable structural features at both field strengths. Residual dipolar effects are responsible for the contrast on these images, rather than frequency changes that scale with magnetic field.
Introduction
Water associated with a collagen matrix (tendons, meniscus, etc.) produces “magic angle” effects on MR imaging(1). The conventional isotropic liquid approach describing T2 relaxation is not sufficient to understand the behavior of transverse magnetization in this situation. The NMR lineshape is not a single (or double) Lorenzian, but rather a more complicated shape that is a strong function of fiber to field orientation and state of hydration (Fig. 1). NMR researchers use anisotropic solvents to provide residual dipolar couplings in biological molecules, allowing the measurement of intramolecular proton distances for structure determination(2). The behavior of water trapped in a collagen matrix can be thought of as a solute in an ordered solvent. Orientation variations of the collagen fibrils, when combined with the longer correlation times of water hydrogen bonded to the protein matrix produce wide and non-Lorentzian linewidths. The non-isotropic averaging of the dipolar interactions on the water molecule results in an apparent ordering direction that points along the collagen fibers and produces the (3cos2ϴ-1) dependence in the frequency dispersion. This has a strong influence on the transverse magnetization (coherence) lifetime. Understanding this dipolar fiber to field mechanism allows interpretation of MR images in collagen containing tissues or even mapping out fiber structure (DAFI technique)(3).Material and Methods
A
short length of frozen human Achilles tendon was immersed in Fomblin and
contained in a10 ml plastic syringe barrel. The tissue fiber direction was
orthogonal to the cylinder axis, allowing rotation in small custom solenoid T/R
coils. This sample rotation allowed images to be obtained as the fiber to field
axis was varied in steps of 30 deg. 3D GRE images were obtained with
0.2 mm isotropic resolution at both 11.7 and 3.0 T using a Bruker BioSpec
117/16USR animal scanner and a GE HDx clinical scanner. Imaging parameters were
identical on both systems: TR 24 ms, TE 4.0 ms. 150x150x150 acq matrix, flip
angle 5 deg, 2 NEX. Images were registered using FLIRT software
(FSL, Oxford). ImageJ software was used
to obtain intensity measurements in selected ROIs on congruent images at 6
orientations and 2 field strengths.Results
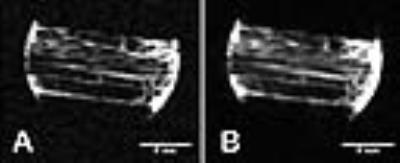
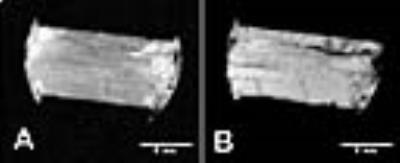
Fig. 2A,2B are 3T and 11.7 T images of the tendon with the field direction parallel (0 deg) to the fiber direction. The tendon has overall low intensity except for some imbedded structures (endotenon, crimped fibers). Rotating the sample to make the fiber to field direction 60 deg produces images having high signal intensity (Fig 3A,3B). Intensity was plotted as a function of rotation angle (Fig. 4). In all orientations the appearance of the 3T and 11.7T images were remarkably similar.Conclusion
What does T2* mean exactly for complex collagen NMR lineshapes(4). It is helpful to keep in mind solid state or liquid crystalline systems where NMR spectra have well defined dipolar couplings. With water hydrogen bonded in the collagen matrix, residual dipolar coupling seem to be the main source of “magic angle” effects seen in MRI. These do not scale with field and are consistent with our data. A recent idea is that T2 varies with orientation when water is entrapped in elliptical nano-cavities(5). It is not clear how this mechanism competes with the anisotropic averaging in a strong hydrogen bonding environment.Acknowledgements
No acknowledgement found.References
1. Krasnosselskaia LV, Fullerton GD, Dodd SJ, Cameron IL. Water in tendon: orientational analysis of the free induction decay. Magn Reson Med 2005;54(2):280-288.
2. Tjandra N, Bax A. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science 1997;278(5340):1111-1114.
3. Szeverenyi NM, Bydder GM. Dipolar anisotropy fiber imaging in a goat knee meniscus. Magnetic Resonance in Medicine 2011;65(2):463-470.
4. Berendsen HJC. Nuclear Magnetic Resonance Study of Collagen Hydration. Journal of Chemical Physics 1962;36(12):3297-3305.
5. Furman GB, Goren SD, Meerovich VM, Sokolovsky VL. Anisotropy of spin-spin and spin-lattice relaxation times in liquids entrapped in nanocavities: Application to MRI study of biological systems. Journal of Magnetic Resonance 2016;263:71-78.
Figures