1926
MR Contrast Effects of Intrinsically Gd-chelated Melanin Nanoparticles at 1.5 and 11.7 Tesla1Radiology, Northwestern University, Chicago, IL, United States
Synopsis
The development of polymeric contrast agents exhibiting a high MR relaxivity has been achieved using bio-inspired metal chelating melanin nanoparticle (Mel NP) synthesized with dopamine or L-3,4-dihydroxyphenylalanine (L-DOPA). In here, we described our simple one-pot synthesis to prepare new Gd chelated Mel NP and their specific features of efficient MR T1 imaging along with their high intrinsic Gd chelation efficiency.
Target Audience
Researchers and/or clinicians with an interest in imaging contrast agent, contrasted-enhanced MRI, high-field MRI systems, and/or applications of nanoparticle imaging probes.Purpose
The development of polymeric contrast agents exhibiting a high MR relaxivity has been achieved using bio-inspired metal chelating melanin nanoparticle (Mel NP) synthesized with dopamine or L-3,4-dihydroxyphenylalanine (L-DOPA). As Mel NP has high affinity and binding capacity to metal ions, Mel NPs are readily doped with various metal ions such as gadolinium (Gd), copper (Cu), and iron (Fe).1-3 However, it is crucial to develop the reliable method to bind metal ions in Mel NPs with higher doping efficiency to potentially improve MR imaging contrast. In our study, one-pot method to synthesize Gd chelated Mel NPs (Gd-Mel NP) was developed. Although most clinical scanners operate below 3 T, clinical high-field MRI systems are becoming increasingly available. Therefore, their r1 relaxivity values were evaluated and compared with conventional metal chelating Mel NP synthesized with post chelating method and commercial available gadopentetate dimeglumine agents for potential MR contrast agent applications.Methods
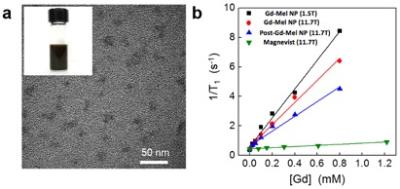
Gd-Mel NPs were prepared by reducing GdCl3 during oxidative polymerization of dopamine hydrochloride with the given molar concentrations of each component. Then, morphologies, size distributions, and metal contents of synthesized materials were characterized with TEM, DLS and ICP-OES. Relaxivities were determined with MRI phantom measurements at both 1.5 T (Magnetom Aera, Siemens Medical Solutions) and 11.7 T (Avance imaging spectrometer, Bruker). Gd-Mel NP was suspended in 1% agarose with various concentrations determined by elemental contents of Gd (0 – 0.8 mM). At 1.5 T and 11.7 T, longitudinal relaxation times (T1) were acquired using progressive saturation method with different repetition time (TR=100 – 4000 ms) for 1.5 T and TR=100 – 10000 ms for 11.7T, respectively. The r1 relaxivity values were determined through the curve fitting of relaxation rate R1 (s−1) versus the Gd component concentration (mM) (Origin 8).Results
The measured diameter of synthesized Gd-Mel NPs in TEM was in the range of 16 – 20 nm which was well matched with hydrodynamic size 16.2 nm in DLS (Fig. 1a). The amount of chelated Gd in Mel NP in our one-pot synthesis was approximately 20 wt% which was 4.7 times higher than that in Gd-Mel NP by post-chelating method. Our one-pot procedure could synthesis an enhanced T1 contrast Gd-Mel NP showing 1.5 fold higher r1 relaxivity at 11.7T than Gd-Mel NP by post-chelating method (Fig. 1b). In different magnetic field MRIs, the r1 relaxivity of Gd-Mel-NP was 9.8 mM-1s-1 at 1.5 T and the r1 relaxivity was decreased to 7.6 mM-1S-1 at 11.7T MRI (Fig. 1b). At 11.7T, the high efficient r1 relaxivity of Gd-Mel NP was confirmed by comparing with measured r1 values (0.4 mM-1S-1) of gadopentetate dimeglumine (Fig. 1b).Discussion
Our synthesized Gd-Mel NPs showed higher loading efficiency than Gd-Mel NP synthesized by post chelated because co-synthesis is more desirable condition for metal ions to be fully reduced by dopamine molecules acting as a reducing agent when oxidative polymerization occurs. The full Gd chelated Mel NP demonstrated effective T1 contrast effects at 1.5 and 11.7T. Although the decreasing r1 of Gd-Mel NP at 11.7T by increasing background T14, our Gd-Mel NP demonstrated significantly higher r1 than commercial available gadopentetate dimeglumine.Conclusion
In Conclusion, we described our simple one-pot synthesis to prepare new Gd chelated Mel NP and their specific features of efficient MR T1 imaging along with their high intrinsic Gd chelation efficiency. Our developed Gd-Mel NP will have a potentially usefulness for MR T1 imaging applications.Acknowledgements
This work was supported by R01CA159178, R21CA173491, R21CA185274, and R21EB017986 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering. This work was also supported by the Center for Translational Imaging.References
1. Enochs, W. S.; Petherick, P.; Bogdanova, A.; Mohr, U.; Weissleder, R., Paramagnetic metal scavenging by melanin: MR imaging. Radiology 1997, 204 (2), 417-23.
2. Felix, C. C.; Hyde, J. S.; Sarna, T.; Sealy, R. C., INTERACTIONS OF MELANIN WITH METAL-IONS - ELECTRON-SPIN RESONANCE EVIDENCE FOR CHELATE COMPLEXES OF METAL-IONS WITH FREE-RADICALS. J. Am. Chem. Soc. 1978, 100 (12), 3922-3926.
3. Morlieras, J.; Chezal, J.-M.; Miot-Noirault, E.; Roux, A.; Heinrich-Balard, L.; Cohen, R.; Tarrit, S.; Truillet, C.; Mignot, A.; Hachani, R.; Kryza, D.; Antoine, R.; Dugourd, P.; Perriat, P.; Janier, M.; Sancey, L.; Lux, F.; Tillement, O., Development of gadolinium based nanoparticles having an affinity towards melanin. Nanoscale 2013, 5 (4), 1603-1615.
4. Hagberg, G. E.; Scheffler, K., Effect of r(1) and r(2) relaxivity of gadolinium-based contrast agents on the T-1-weighted MR signal at increasing magnetic field strengths. Contrast Media Mol I 2013, 8 (6), 456-465.