1924
Accurate Tissue Oxygen Level-dependent MRI with true T1-weighted signalSoHyun Han1, HyungJoon Cho2, and Seong-Gi Kim1,3
1Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon, Korea, Republic of, 2Department of Biomedical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
Tissue oxygen level dependent (TOLD) MRI utilizes that T1 is directly related with tissue pO2. TOLD MRI is acquired by gradient echo based with a minimized TE. However, T2* contribution may not fully be suppressed. Here, we investigated the modulations of longitudinal and transverse relaxation times with oxygen challenge (OC) and compared TOLD signals from FLASH, UTE, and TSE at 7 T and 15.2 T. At both magnetic fields, T2 did not change with tissue pO2 while T1 did. Spin echo and UTE appear to reflect true pO2 levels due to invariability in T2 during OC and minimized T2* with ultrashort TE, respectively.
Purpose
The utilization of inhaled oxygen as an MR contrast agent is appealing, due to the fact that it is truly noninvasive and avoids the administration of exogenous contrast agents1. Several studies have showed the feasibility of utilizing the blood oxygen level-dependent (BOLD) effect as a surrogate of tissue pO22-4. However, it is generally known that BOLD signal is also affected by local hematocrit, vascular volume, pH, flow, and vessel density5. Tissue oxygen level-dependent (TOLD) MRI utilizes T1-contrast, as T1 is directly related with tissue pO26-7. Generally, TOLD MRI is acquired by a gradient echo based fast low-angle shot (FLASH) sequence with a minimized echo time (TE). On the other hand, the T2* contribution may not fully be suppressed with FLASH acquisition especially at ultra-high magnetic fields. In this regard, we have investigated the modulations of longitudinal and transverse relaxation times with oxygen inhalation challenge and compared the TOLD signals from FLASH, ultra-short echo time (UTE), and turbo spin echo (TSE) acquisitions at 7 T and 15.2 T.Methods
MR experiments were performed on 7 T/16-cm and 15.2 T/11-cm MRI systems (Bruker BioSpin, Billerica, MA, Paravision 6). A total of 6 male Sprague-Dawley rats (250-300 g, 7 weeks of age) were used with approval from the Institutional Animal Care and Use Committee of Ulsan National Institute of Science and Technology for 7 T and Sungkyunkwan University for 15.2 T. First, T1, T2, and T2* mappings were performed at room air (RA) conditions (30% oxygen) and 5 mins post-oxygen challenge (OC) conditions (100% oxygen). Second, to determine TE-dependent TOLD signal changes, multi-echo spin echo and gradient echo measurements (MSME, MGE, respectively) were performed during the oxygen challenge. Finally, TOLD MRI was performed with FLASH, UTE, and TSE during OC with the following parameters at 15.2 T: TE/TR = 2.2/30 ms, flip angle = 30° (FLASH), TE/TR = 0.31/30 ms, flip angle = 30° (UTE), and TE/TR = 4.7/200 ms (TSE), the matrix size of 96 × 96, field of view (FOV) of 30 × 30 mm2, number of slices of 2, and slice thickness of 1.5 mm. The temporal paradigm for OC was 5 min (RA) – 5 min (OC) – 5 min (RA).Results
Fig. 1(A) and (B) show T1, T2, and T2* maps of one representative animal under RA (30% oxygen) and OC (100% oxygen) at 7 T and 15.2 T, respectively. T1 shortening was observed at both magnetic fields while T2* varied but T2 did not significantly vary at both magnetic fields. To investigate potential contamination of transverse relaxation effect to TOLD signal, dynamic changes of TE-dependent gradient echo and spin echo MRI responding to OC were plotted in Fig. 2(A) and (B), respectively. The signal of FLASH under OC modulated with TE while those of TSE did not change. In Fig. 3, TOLD MRI images were shown for FLASH, UTE, and TSE. The UTE image was less susceptible to the air tissue interface region compared to the FLASH image. The dynamic signal change of the cortex region within red square box was shown for FLASH, UTE, and TSE acquisitions. Clearly, FLASH is the least sensitive to OC due to greater T2* effect compared to UTE.Discussion and conclusion
At both magnetic fields of 7 T and 15.2 T, T2 did not change with tissue pO2 while T1 did change with tissue pO2. By taking advantage of this observation, we demonstrated the feasibility of TOLD MRI with a T1-weighted spin-echo based sequence with contrast due to TE independency. The exact reason for T2 independency to oxygen is not clear but likely due to the counteraction of the T2 decrease from paramagnetic oxygen by the T2 increase from an increase in the oxygen saturation of hemoglobin. The spin echo based sequence with short TR has a lower SNR compared to gradient echo based sequences with the same TR. Additionally, the signal change was higher for spin echo acquisition which agrees with simulations performed using MR parameters and values from Fig. 1 (data not shown). At ultra-high fields TOLD MRI with FLASH acquisition does not reflect pO2 response accurately due to T2* contribution. Spin echo and UTE acquisitions appear to reflect true pO2 levels due to invariability in T2 values during OC and minimized transverse relaxations with ultrashort TE acquisition, respectively.Acknowledgements
This work was supported by IBS-R015-D1.References
[1] Hallac RR, Zhou H, Pidikiti R, Song K, Stojadinovic S, Zhao D, Solberg T, Peschke P, Mason RP. Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magn Reson Med 2014;71:1863-1873. [2] Zhao D, Jiang L, Hahn EW, Mason RP. Comparison of 1H blood oxygen level-dependent (BOLD) and 19F MRI to investigate tumor oxygenation. Magn Reson Med 2009;62:357-364. [3] Elas M, Williams BB, Parasca A, et al. Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magn Reson Med 2003;49:682-691. [4] Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med 2002;48:980-986. [5] Baudelet C, Gallez B. Current issues in the utility of blood oxygen level dependent MRI for the assessment of modulations in tumor oxygenation. Med Imaging Rev 2005;1:229-243. [6] O’Connor JPB, Naish JH, Jackson A, et al. Comparison of normal tissue R-1 and R-2* modulation by oxygen and carbogen. Magn Reson Med 2009;61:75-83. [7] Thulborn. KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta 1982;714:265-270.Figures

(A),
(B) T1, T2, T2* maps (30% oxygen – upper row and 100%
oxygen – bottom row) at 7T, 15.2T systems, respectively.
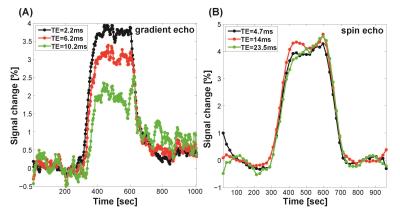
Dynamic
changes of TE dependent gradient echo (A) and spin echo (B) MRI responding to
OC. The signal change of gradient echo under OC modulated with TE while those
of spin echo did not change.
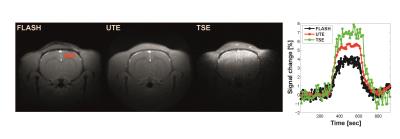
TOLD
MRI images for FLASH, UTE, and TSE. The dynamic signal changes of the cortex
region depicted in TOLD image for FLASH as red square box. Black, red, and
green lines represent the dynamic signal changes for FLASH, UTE, and TSE.