1912
Measurement of Murine Single-Kidney Glomerular Filtration Rate using Dynamic Contrast Enhanced MRI1Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, United States, 2Division of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN, United States
Synopsis
A method for noninvasive assessment of mouse glomerular filtration rate (GFR) using dynamic contrast enhanced MRI (DCE-MRI) was developed and validated. The kinetics of gadolinium in the abdominal aorta and two kidneys were measured using a snapshot fast low angle shot based T1 mapping method with a temporal resolution of 1 s. A modified bi-compartmental model was used for quantification of GFR by a least-squares fitting. As a reference standard, GFR was also measured using FITC-inulin clearance. Single-kidney GFR measured from both methods showed a good agreement, suggesting the proposed DCE-MRI method provides an accurate measurement of murine single-kidney GFR.
Introduction
Glomerular filtration rate (GFR) is a fundamental index for diagnosis of renal diseases and evaluation of treatment interventions. Gadolinium (Gd)-based dynamic contrast enhanced MRI (DCE-MRI) provides an important tool for noninvasive measurement of single-kidney GFR1,2. However, its utility in mice at high-field strength has not been shown. In this study, we aimed to develop and validate a method for measurement of murine single-kidney GFR using DCE-MRI at 16.4 T.Methods
DCE-MRI Study. This study was approved by the Institutional Animal Care and Use Committee. MRI studies were performed on a vertical 16.4 T Bruker scanner equipped with a 38-mm inner diameter birdcage coil. Seven male 129S1 mice 4 weeks after either unilateral sham (n=4) or renal artery stenosis (RAS, n=3) surgeries were used. A saturation recovery T1 mapping method with snapshot fast low angle shot readout was developed to trace Gd dynamics with a temporal resolution of 1 s. Two slices located at the center of both kidneys were imaged. Following acquisition of the proton density (M0) and ten baseline T1-weighted (Mt) images, 37.5 mM gadodiamide (0.03mmol/kg) was injected through the tail-vein within 2 s, after which the Mt images were acquired repetitively. Other imaging parameters were: recovery time, 0.25 and 0.57 s; encoding scheme, central; TR 4.92 ms; TE 0.8 ms; flip angle 15°; slice thickness 2 mm; FOV 2.56×2.56 cm2; matrix size 128×128; number of repetitions 150. T1 maps were generated by mono-exponential fitting and the change in R1 (ΔR1) from baseline was used as an index of Gd concentration.
In addition, kidney volume was measured using a three-dimensional fast imaging with steady precession sequence, and renal perfusion using a flow-sensitive alternating inversion-recovery sequence with rapid acquisition with relaxation enhancement, as described previsouly3.
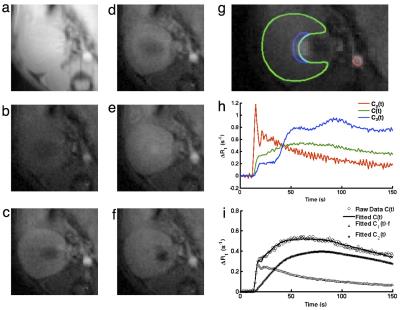
Compartmental Model. The compartmental model is shown in Fig. 1. The kidney is compartmentalized into plasma and tubules. In this model, C1(t) is the Gd concentrations in renal plasma and C2(t) is the Gd concentration in the tubules within the whole kidney. Changes in C1(t) and C2(t) can be described by the following kinetic equations:
dC1(t)/dt=Ktrans(Cp(t)-C1(t))
dC2(t)/dt=k1C1(t)-k2C3(t)
where Ktrans is the perfusion rate constant, Cp(t) the arterial input function (AIF), k1 the normalized GFR, k2 the Gd efflux rate, and C3(t) the concentration of filtered Gd in the inner medullary collecting ducts (IMCD) space. In image analysis, the IMCD space was prescribed at the innermost part of medulla that did not show the blooming effect of Gd. The total Gd concentration in kidney is C(t)=C1(t)×f+C2(t), where f is the volume fraction of renal plasma space. Dynamic curves were model-fitted with the Levenberg-Marquardt least-squares algorithm. Then GFR (ml/min) was calculated as k1×V×60, where V is the effective kidney volume.
GFR by FITC-inulin Clearance. One day after MRI, GFRs of the same mice were measured by FITC-inulin clearance4. 3.5% FITC-inulin 3.74 ml/g was injected retro-orbitally, after which ~20ml blood was repeatedly collected via the saphenous vein at 3, 7, 10, 15, 35, 55, and 75 minutes. The concentration of FITC-inulin in isolated plasma was measured using fluorescence, with 485 nm excitation and 538 nm emission. A two-compartment model was used, in which the concentration of FITC-inulin was described by A×e-at + B×e-bt, with the first and second term represents redistribution and clearance, respectively. GFR was then calculated as I/(A/a+B/b), where I is the total amount of injected FITC-inulin. Single-kidney GFR was quantified by partitioning the total GFR based on the blood flow (perfusion × volume) of each kidney.
Results
Fig. 2 a-f shows representative M0 (Fig. 2a) and Mt images acquired at baseline (Fig. 2b) and 5, 17, 73, and 138 s post-Gd injection (Fig. 2c-f). Manually traced regions of interest (ROIs) for the abdominal aorta (red), kidney (green), and IMCD (blue) are shown in Fig. 2g. The corresponding time-intensity curves of Gd concentration in all three ROIs are shown in Fig. 2h, and the model-fitted curves in Fig. 2i.
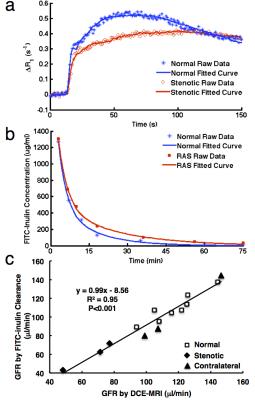
Representative raw data and model-fitted Gd dynamics in normal and stenotic kidney are shown in Fig. 3a. The attenuated stenotic kidney curve suggests impaired perfusion and filtration, which was also reflected in the slower clearance of FITC-inulin from plasma (Fig. 3b). A good correlation was found between GFRs measured by DCE-MRI and FITC-inulin clearance of normal, stenotic, and contra-lateral kidneys (Fig. 3c).
Conclusion
This study proposes a method for noninvasive measurement of murine single-kidney GFR using DCE-MRI. The close curve fitting in DCE-MRI and the good correlation between GFRs measured by DCE-MRI and FITC-inulin clearance suggest that our method can reliably measure single-kidney GFR in mice.Acknowledgements
None.References
1. Lee VS, Rusinek H, Bokacheva L, et al. Renal function measurements from MR renography and a simplified multicompartmental model. Am. J. Physiol. Renal Physiol. 2007;292:F1548–F1559.
2. Annet L, Hermoye L, Peeters F, Jamar F, Dehoux J-P, Van Beers BE. Glomerular filtration rate: assessment with dynamic contrast-enhanced MRI and a cortical-compartment model in the rabbit kidney. J. Magn. Reson. Imaging 2004;20:843–9.
3. Jiang K, Ferguson CM, Ebrahimi B et al. Noninvasive Assessment of Renal Fibrosis with Magnetization Transfer MR Imaging: Validation and Evaluation in Murine Renal Artery Stenosis. Radiology, 2016, Ahead of Print, 10.1148/radiol.2016160566
4. Qi Z, Whitt I, Mehta A, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286(3):F590-6.
Figures