1908
Feasibility and value of View-shared Compressed Sensing combined fast DCE-MRI in quantitative evaluation of unilateral renal embolism in rabbitsHanjing Kong1, Bin Chen2,3, Hao Li1,4, Bihui Zhang5, Haochen Wang5, Xiaodong Zhang6, Min Yang5, Jue Zhang1,2, Xiaoying Wang1,6, and Jing Fang1,2
1Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, People's Republic of China, 2College of Engineering, Peking University, Beijing, People's Republic of China, 3Department of technical research and development, Instrumentation Technology and Economy Institute, Beijing, People's Republic of China, 4Department of Radiology, University of Cambridge, United Kingdom, 5Interventional radiology and vascular surgery, Peking University First Hospital, Beijing, People's Republic of China, 6Department of Radiology, Peking University First Hospital, Beijing, People's Republic of China
Synopsis
Dynamic-contrast enhanced MR imaging is widely employed as a clinical tool in kidney imaging and renal function measurements. Some novel works have been made in improve temporal resolution. In this work, we adopt a 3D Cartesian MRI with compresses sensing and variable view sharing sequence to explore its evaluation in renal embolism assessment. GFR was calculated and renal embolism was confirmed by histological results. Fast DCE-MRI is a promising method for renal embolism diagnose.
Introduction
Dynamic-contrast enhanced MR imaging (DCE-MRI) is widely employed as a clinical tool in kidney imaging and renal function measurements. However, due to limitations in temporal/spatial resolution of the traditional imaging sequence, the temporal resolution is not adequate in quantitative measurement. Recently, a 3D Cartesian MRI with compresses sensing and variable view sharing sequence has been proposed to accelerate image acquisition [1]. In this study, we aim to validate the feasibility and value of the proposed sequence in quantitative renal measurements in rabbits with unilateral renal embolism. And further compared the association of DCE derived quantitative parameters with the histologic findings.Methods
This study was approved by the Hospital Ethics Committee. Twenty New Zealand white rabbits (male, 2.5–3.0 kg) were implemented a unilateral renal embolism surgery procedure after anesthetization and were enrolled in dynamic contrast-enhanced MR imaging examinations. DCE-MRI data were acquired using a GE 3.0T MR scanner (Signa ExciteTM; General Electric Medical Systems, Milwaukee, WI, USA) with 8-channel TORSOPA coil. Before DCE-MRI acquisition, data for constructing T1 map were acquired with the identical parameters using three flip angles (3, 9, 20)° method [2]. A view-shared compressed sensing combined fast DCE-MRI sequence was applied with the following parameters: TR = 4.0 msec / TE = 1.5 msec, flip angle = 12°, acquisition matrix = 128× 128, NEX=1, slice thickness = 4 mm, slices = 16, field of view = 380mm. Five frames of non-enhanced volumes of the kidney were acquired before the bolus administration and acquisition time was 3 seconds per frame. Then, 0.1 mmol / kg body weight of Gd-DTPA (Magnevist, Bayer Schering Pharma AG, Berlin, Germany) and 5 ml saline was successively injected at 3 ml / sec. Images were acquired immediately and totally 120 frames were acquired in about six minutes. According to fig1, the k-space points were first recombined by view sharing the adjacent data sets to generate reconstruction data VCSD, then compressed sensing reconstruction [3] was carried out. The two compartmental model [4] was used in quantitative parameters calculating. A 3×3 voxel ROI within the abdominal aorta distal to the branch of the renal artery was drawn to generate the arterial input function (AIF). The tissue signal intensity curves were generated from the reconstructed images. Then the signal intensity-time curves were converted into Gd-concentration curves. Renal glomerular filtration rate and Vb were calculated.Results
The dynamic inflow of contrast agent in the abdominal arterial, renal cortex, medulla and late venous phases is easily discernible. AIF and tissue signal intensity curves for normal kidney are in line with existing study [5]. The tissue signal intensity of medulla on embolism kidney was lower than that of normal kidney, while signal intensity of cortex are very close between embolism and normal kidney. In quantitative measurements shown in Fig. 3, mean GFR of right and left kidney were 1.49±0.96 ml/min, 3.17±1.22 ml/min, respectively. Large decrease of GFR and Vb were found in the right kidneys while the right kidneys remain normal. Regions with extremely low GFR and Vb (red arrow) (GFR = 0.86 ± 0.05 ml/min, 0.77 ± 0.09 ml/min, Vb = 0.14 ± 0.04, 0.19 ± 0.07) showed significant difference with symmetrical regions of normal kidney (GFR = 2.85 ± 0.16 ml/min, 3.04. ± 0.43 ml/min, Vb =0.31± 0.10, 0.35±0.08, P=0.05) And had proved embolism by tissue specimen(fig3. (c)). The glomeruli show ischemic and wrinkled features with thickening change of Bowman‘s capsule and necrosis of the renal tubular epithelial cells is observed. The basement membrane is exposed. The brush border of some tubular epithelial cells fell off. The tubular epithelial cells become flat and tubular lumen expands. Renal interstitial fibrosis can be seen.Discussion and Conclusion
DSA is gold standard to diagnose renal embolism disease. However, it expose patients to radiation, which can increase the risk of cancer over time. DCE-MRI with high temporal resolution can quantitatively calculate kinetic parameters, which has been extensively used in renal dieases. To the best of our knowledge, almost no prior studies were focused on evaluating the renal embolism with DCE-MRI. Tissue signal intensity curve of medullar and quantitative assessment of GFR and Vb showed significant reduction in the embolism region and has been proved by tissue specimen and histologic results. The View-shared Compressed Sensing combined fast DCE-MRI derived quantitative parameters may be a promising imaging biomarker of renal embolism evaluation.Acknowledgements
No acknowledgement found.References
1. Levine E, Daniel B, Vasanawala S, et al. 3D Cartesian MRI with compressed sensing and variable view sharing using complementary poisson-disc sampling.[J]. Magnetic Resonance in Medicine, 2016. 2. Chen B, Zhang Y, Song X, et al. Quantitative Estimation of Renal Function with Dynamic Contrast-Enhanced MRI Using a Modified Two-Compartment Model[J]. Plos One, 2014, 9(8):e105087-e105087. 3. Lustig M, Donoho D, Pauly J M. Sparse MRI: The application of compressed sensing for rapid MR imaging[J]. Magnetic Resonance in Medicine, 2007, 58(6):1182-1195. 4. Tofts P S, Cutajar M, Mendichovszky I A, et al. Precise measurement of renal filtration and vascular parameters using a two-compartment model for dynamic contrast-enhanced MRI of the kidney gives realistic normal values[J]. European Radiology, 2012, 22(6):1320-1330. 5. Chen B, Kai Z, Bo L, et al. High temporal resolution dynamic contrast-enhanced MRI using compressed sensing-combined sequence in quantitative renal perfusion measurement[J]. Magnetic Resonance Imaging, 2015, 33(8):962-969.Figures
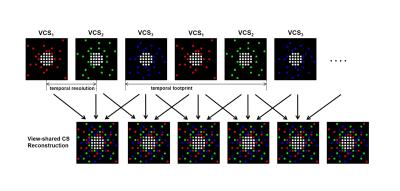
Figure1. Diagram of reorganizing
and reconstruction of k-space data.

Figure 2 . Typical (a) arterial
inflow function curve and (b) tissue intensity curve of cortex (blue) and
medulla (green) of normal kidney, and (c) tissue intensity curve of cortex
(blue) and medulla (green) of normal kidney (solid lines) and embolism kidney
(dotted lines).
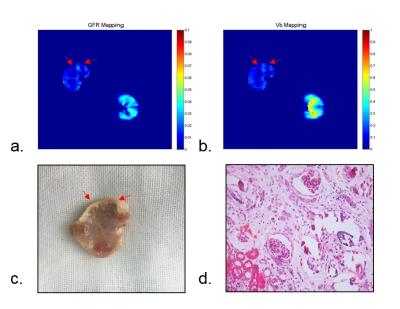
Figure 3. Typical (a) GFR
mapping(ml/min), (b) Vb mapping, (c) tissue specimen and (d) histological
section. Ischemic and wrinkled features with thickening change
of Bowman‘s capsule; necrosis of the renal tubular
epithelial cells and basement membrane is exposed.