1888
Simultaneous Multi-Slice Cardiac ASL1Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Electrical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
Cardiac arterial spin labeling (ASL) is a promising technique for the quantification of myocardial blood flow (MBF) and has been shown to detect clinically relevant changes in myocardial perfusion under vasodilator stress. However, current cardiac ASL techniques have limited spatial coverage because they cannot be repeated for multiple slices due to limited duration of pharmacologically induced peak stress (~3 min). In this work, we demonstrate the feasibility of using blipped CAIPI bSSFP for cardiac FAIR ASL.
Background
Cardiac arterial spin labeling (ASL) is a promising technique for the quantification of myocardial blood flow (MBF) and has been shown to detect clinically relevant changes in myocardial perfusion under vasodilator stress.1 However, current cardiac ASL techniques have limited spatial coverage because they cannot be repeated for multiple slices due to limited duration of pharmacologically induced peak stress (~3 min). Simultaneous multi-slice (SMS) using CAIPIRINHA or blipped CAIPI are attractive methods to increase slice coverage because multiple slices are simultaneously acquired without increasing scan time. However, CAIPIRINHA has an unfavorable tradeoff between the effective off-resonance profile and multiband acceleration factor for bSSFP imaging .2,3 Alternatively, blipped CAIPI uses gradients to achieve interslice shifts without changing the off-resonance behavior and is therefore more suitable for bSSFP imaging.4 In this work, we test the feasibility of using blipped CAIPI SSFP for cardiac FAIR ASL.Method
Blipped-CAIPI bSSFP – A FOV/n interslice image shift was achieved by cycling through n Gz phase encoding gradients in the slice direction that were applied simultaneously with conventional Gy phase encodes. The difference in gradient area between adjacent Gz phase encodes is calculated as, $$$ \Delta A = 2\pi/(\gamma n z_{gap}) $$$ where $$$z_{gap} $$$ is the slice separation and n is the desired FOV/n interslice shift. Phase encodes were acquired using an interleaved view order that grouped Gz phase encodes together to reduce the number of k-space jumps to minimize eddy current artifacts. Images were reconstructed using split-slice GRAPPA5 to minimize interslice leakage with a kernel size of [5x5] and coil combined using optimal B1 coil combination.6 Sensitivity maps were estimated from fully sampled reference images.
Imaging – All images were acquired on a 3T GE scanner (Signa Excite HD) with an 8-channel cardiac coil. In four healthy volunteers (29 ± 2.6 yo, 3M, 1F), two short axis cardiac images were acquired during mid-diastole at a mb factor of 2 (FOV/2 shift) and a 2.5 cm slice gap with TR/TE = 3.8/1.6 ms, FA = 300, matrix size = 128x128, and partial Fourier factor = 6/8. FAIR ASL was performed using a selective inversion slab of 6 cm, symmetrically spaced about the two acquired slices for control acquisitions and a non-selective inversion for labeled acquisitions. The corresponding single slice FAIR references were acquired for comparison. MBF, physiological noise (PN), and temporal SNR (TSNR = MBF/PN) was estimated within a ROI corresponding to the left ventricle, as described by Zun et. al.7
SNR Analysis – SNR and g-factor maps were calculated on the acquired baseline (no ASL contrast) images. G-factor maps were estimated using the pseudo-replica approach.8
Results & Discussion
Figure 1 contains representative SMS images from a single volunteer with corresponding SNR and g-factor maps. The average g-factor loss within the myocardium was 1.10 ± 0.13. G-factor losses of up to 1.45 were observed in the lateral wall and can be attributed to low number of coil elements and the lack of coil diversity in the slice direction. As shown in Table 1, MBF estimates from SMS FAIR were 1.65 ± 0.69 ml/g/min, 0.67 ± 0.30 ml/g/min for the basal and mid short axis slices, respectively. This was comparable to single slice FAIR with 1.06 ± 0.30 ml/g/min, 1.04±0.23 ml/g/min respectively. Figure 2 contains a representative MBF and TSNR map. We observed lower TSNR in SMS as well as single slice FAIR of 3.17 and 5.08 respectively for the mid-short axis slice, compared to previous studies.7 We attribute this to the longer imaging window and a thickened inversion slab.Conclusion
We demonstrate the feasibility of using blipped CAIPI bSSFP imaging for cardiac ASL using FAIR. Compared to single-slice FAIR, the proposed method is capable of acquiring two slices simultaneously with comparable estimates of MBF. Extension to more than two slices remains future work, and will likely require the use of higher density receiver arrays (>8 elements) that provide diversity in the slice direction.Acknowledgements
National Institutes of Health #R01-HL130494; American Heart Association #13GRNT13850012; Wallace H. Coulter FoundationReferences
1. Zun Z, Varadarajan P, Pai RG, Wong EC, Nayak KS. Arterial spin labeled MRI detects clinically relevant increases in myocardial blood flow with vasodilatation. Journal of Cardiovascular Magnetic Resonance. 2011;13(Suppl 1):O94.
2. Stäb D, Ritter CO, Breuer FA, Weng AM, Hahn D, Kostler H. CAIPIRINHA accelerated SSFP imaging. Magnetic Resonance in Medicine. 2011;65:157–164.
3. Landes V, Jao TR, Nayak KS. CAIPIRINHA-SSFP with improved banding artifact performance. ISMRM Workshop on Simultaneous Multi-slice Imaging 2015, Pacific Grove, July 2015.
4. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-Controlled Aliasing in Parallel Imaging (blipped-CAIPI) for simultaneous multi-slice EPI with reduced g-factor penalty. Magnetic Resonance in Medicine. 2012;67(5):1210-1224.
5. Cauley SF, Polimeni JR, Bhat H, Wang D, Wald LL, Setsompop K. Inter-slice Leakage Artifact Reduction Technique for Simultaneous Multi-Slice Acquisitions. Magnetic Resonance in Medicine. 2014;72(1):93-102.
6. Roemer PB, Edelstein, WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magnetic Resonance in Medicine. 1990;16:192–225.
7. Zun Z, Wong EC, Nayak KS. Assessment of myocardial blood flow (MBF) in humans using arterial spin labeling (ASL): Feasibility and noise analysis. Magnetic Resonance in Medicine. 2009;62: 975–983.
8. Robson PM, Grant AK, Madhuranthakam AJ, Lattanzi R, Sodickson DK, McKenzie CA. Comprehensive Quantification of Signal-to-Noise Ratio and g-Factor for Image-Based and k-Space-Based Parallel Imaging Reconstructions. Magnetic Resonance in Medicine. 2008;60(4):895-907.
Figures
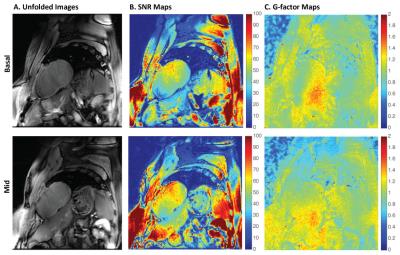
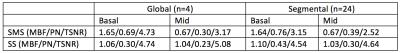
Table 1: Myocardial blood flow (MBF), physiological noise (PN), and temporal SNR (TSNR) reported in SMS and single slice (SS) FAIR ASL. SMS ASL has lower TSNR and higher physiological noise than SS ASL due to g-factor losses. MBF and PN are reported in units of ml/g/min.
