1876
Signal behavior of Ultra-High-b radial DWI (UHb-rDWI) signal in different tract of the cervical spinal cord1Utah Center for Advanced Imaging Research, University of Utah, Salt Lake City, UT, United States, 2Department of Physics and Astronomy, University of Utah, Salt Lake City, UT, United States, 3Department of Neurology, University of Utah, Salt Lake City, UT, United States, 4Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
Synopsis
The ultrahigh-b radial DWI (UHb-rDWI) technique is used to study the white matter disease in the spinal cord. The diffusion signal from the extra axonal (EA) space drops to noise level while that from the intra axonal (IA) space is almost constant at UHb region for the myelinated axons where the myelin layers prohibit the exchange of water molecules between IA and EA spaces. However for partially or unmyelinated axons, the diffusion signal from IA space is no longer constant. The signal behavior at UHb region could be used as a biomarker for the demyelination and axonal loss.
INTRODUCTION
Injury to the spinal cord may include local demyelination and/or axonal damage and lead to varying degrees of neurologic deficit, which can cause persistent disability. Imaging biomarkers for earlier disease detection and non-invasive monitoring in the follow-up and treatment stages would be significant advancement in the care of patients. Magnetic resonance imaging (MRI) enables superb contrast and spatial resolution of the spinal cord; however, conventional MRI sequences (T1- and T2-weighted) are limited in their ability to detect the very early stages of disease when clinical symptoms may be vague1. The emerging advanced high-b diffusion-weighted imaging (DWI) technique provides better contrast between white matter (WM) and gray matter (GM). In the present study, we used an ultrahigh-b radial DWI (UHb-rDWI) technique2 in which UHb diffusion weighting gradients are applied in the direction perpendicular to the cord. This technique greatly reduces the geometric distortion due to susceptibility difference between the cord and vertebral bones by implementing reduced field of view (FOV) in the phase encoding direction and the motion induced artifact (respiratory and cardiac) using single shot acquisition. The radial diffusivity depends strongly on diffusion time, b-value and relative concentration of intra axonal (IA) and extra axonal (EA) spaces. Our previous finding based on Monte Carlo simulation3 demonstrated that the signal from the EA space of healthy spinal cord, where the motion of water molecule is hindered, decays and drops to noise level while that from the IA space, where the motion is restricted, remains constant in UHb zone. The purpose of this paper is to study the signal behavior of the UHb-rDWI signal at different tracts of the cord and validate the reproducibility of the technique.METHODS
After approval from local institutional review board and informed consent, one healthy volunteer and one multiple sclerosis patient with an intramedullary lesion at c2-c3 level underwent UHb-rDWI experiment twice in the interval of 4 month on a Siemens 3T MRI system (Trio, Siemens Medical Solutions, Erlangen, Germany). Firstly, T2-weighted images were acquired for planning the UHb-rDWI experiment. Then axial high-b diffusion images were acquired using 2D Single Shot Diffusion-Weighted Stimulated EPI with Reduced FOV (2D ss-DWSTEPI-rFOV) sequence and 8 channel array coil4. The imaging parameters were TR/TE=3 s/64 ms, FOV read/phase=128/44 mm, 6 min 19 sec scan time, 6 averages, 21 slices, 1x1x4 mm3 voxel dimension, and linearly spaced 7 b-values (12 ms gradient duration and 38 mT/m amplitude) ranging from 573 to 7,348 s/mm2 along the left-right direction corresponding to the 7 mixing times, TMs (time interval between two 90° RF pulses) ranging from 9 to 465 ms. An additional bo image without diffusion gradient was obtained for each TM for correcting T1 decay during TM.RESULTS
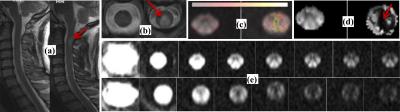
The sagittal and axial (volunteer-left, patient-right) T2 weighted images of the volunteer and the patient with lesion at c2-c3 level (red arrow) are shown in figs. 1a and 1d, respectively The slice at c2 vertebra was chosen for data analysis. Fig. 1(c-d) illustrate the maps of high-b decay rate constant, DH and IA fraction, IAF ( volunteer-left, patient-right), respectively. The value of DH is markedly increased while IAF is markedly reduced (red arrow) in the patient. Fig. 1e shows the series of UHb-rDWI (top series: volunteer and bottom: patient) corresponding to the 7 b-values. The diffusion curves obtained from different region of the slice are shown in figs. 2(a-d). The upper and lower two curves correspond to the two scans for volunteer and patient, respectively.DISSUSSIONS and CONCLUSIONS
The diffusion signal from the EA space decays while that from the IA space is almost constant with increasing b-values for the myelinated axons where the myelin layers prohibit the exchange of water molecules between IA and EA spaces. However for partially or unmyelinated axons, the diffusion signal from IA space is no longer constant. The plots of fig. 2 demonstrate this fact. The plateau of diffusion curve in the lesion of patient’s spinal cord is remarkably lower than that in a corresponding location in the volunteer due to the leakage from IA space. The diffusion curves between the patient and volunteer are similar in healthy regions of the spinal cord (not shown). This fact is also depicted in the DH and IAF maps which could be used as a biomarker for the demyelination and axonal loss. The plateau of the curve corresponds the axonal density, which is different at different tracts within a spinal cord section. The identical diffusion curves for the two scans of each subject confirm the reproducibility of the technique.Acknowledgements
This work is supported by VA Merit Review Grant and NMSS Research Grant (RG 5233- A- 2).References
[1]. Bergers E, Bot JCJ, De Groot CJ et al. Axonal damage in the spinal cord of MS patients occurs largely independent of T2 MRI lesions. Neurol. 2002;59(11):1766–1771.
[2] Sapkota N, Shi X, Shah LM, et al. Two-dimensional single-shot diffusion-weighted stimulated EPI with reduced FOV for ultrahigh-b radial diffusion-weighted imaging of spinal cord. MRM. May 19, 2016. doi: 10.1002/mrm.26302. Accessed June 14, 2016.
[3] Sapkota N, Yoon S, Thapa B, et al. Characterization of Spinal Cord White Matter by Suppressing Signal from Hindered Space. A Monte Carlo Simulation and an Ex Vivo Ultrahigh-b Diffusion-Weighted Imaging Study. JMR. 2016;272:53-59.
[4] Sapkota N, Thapa B, Lee YJ et al. Eight-channel decoupled array for cervical spinal cord imaging at 3T: six-channel posterior and two-channel anterior array coil. CMRB 2016;46B:90-99.
Figures