1871
Diffusion MRI and magnetic resonance spectroscopy reveal microstructural and functional alteration in chronic mild stress exposed rat brains: A CMS recovery studyAhmad Raza Khan1, Brian Hansen1, Ove Wiborg2, Christopher D Kroenke3, and Sune N Jespersen1,4
1Clinical Medicine, Center of Functionally Integrative Neuroscience, Aarhus, Denmark, 2Clinical Medicine, Translational Neuropsychiatry Unit, Risskov, Denmark, 3Advanced Imaging Research Center, Portland, OR, United States, 4Department of Physics and Astronomy, Aarhus, Denmark
Synopsis
Chronic mild stress (CMS) exposure leads to depression and other psychiatric disorder. Temporal changes post CMS exposure is still unclear. Present study employed CMS exposure on rats and utilised in-vivo longitudinal diffusion MRI and magnetic resonance spectroscopy (MRS) to reveal microstructural and functional alterations up to eight weeks post CMS exposure. Advanced diffusion kurtosis metrics have revealed significant alteration in stress sensitive regions, such as amygdala, hippocampus, prefrontal cortex and caudate putamen. MRS also showed significant metabolic alteration in ventral hippocampus, particularly on week1 post CMS exposure. Present finding could be useful in treatment of depression or similar disorders.
INTRODUCTION
Chronic mild stress (CMS) exposure leads to depression and other psychiatric disorder [1, 2]. An unpredictable CMS exposure on rats is considered a realistic model of depression and may help to elucidate mechanisms underlying depression and its effect on brain microstructure and chemistry [2, 3]. CMS induces microstructural and functional alterations mainly in hippocampus, amygdala and prefrontal cortex [4, 5]. However, the mechanisms governing reversal of these alterations are still unclear and the timeline for recovery is vague. An improved understanding of these mechanisms would have clinical significance in treatment and management of depression and/or similar psychiatric disorders. The present study aims to explore microstructural and functional alterations longitudinally in rat brains over eight weeks after cessation of CMS exposure. The study utilized recently developed diffusion kurtosis MRI metrics [6, 7] and magnetic resonance spectroscopy (MRS) of ventral hippocampus.METHODS
Long Evans rats were exposed to an eight weeks long CMS paradigm. Following this, sucrose consumption identified a group of rats as anhedonic [2]. Anhedonic rats (n=8) were scanned on week1, week3 and week 8 post CMS exposure. Age matched controls (n=8) underwent the same scan protocol at weeks 1 and 5. All MR experiments were performed on a 9.4 T preclinical animal MR system (Bruker Biospec, Bruker Biospin, Germany). Rats were anesthetized using gas anaesthesia (Isoflurane: 1.5-2.5%). Animal body temperature and respiration rate was monitored throughout the study using physiological monitoring system. Rats were scanned with imaging system equipped BGA-12HP gradients and a cross-coil setup with a 76 mm quadrature coil for excitation and a four element rat brain cryo-surface coil for reception (Bruker Biospin Ettlingen, Germany). Diffusion MRI data was acquired with diffusion protocol consisted of a fast kurtosis acquisition (1-9-9)[7] using a segmented EPI sequence (four segments) with parameters: diffusion times (Δ/δ) of 14ms/6ms, b-values 1.0 ms/µm2 and 2.5 ms/µm2; 3 b=0 images were acquired. Scan parameters are: TR/TE=2237/27 ms, 38 slices, 300 µm isotropic resolution, matrix size 128x64 and 20 averages were acquired resulting in a total scan time of 1h 10m. Diffusion tensor metrics (D ̅, D∥ and D⊥), axially symmetric metrics (K∥, K⊥ and K ̅ ) and fast kurtosis metrics (W∥, W⊥ and W ̅) were computed from diffusion MRI data. Proton magnetic resonance spectroscopy data was collected from left ventral hippocampus using PRESS (Point resolved spectroscopy sequence) using TR/TE 5000/16 ms. All spectroscopy data was acquired with linewidth <20 Hz (~0.05 ppm). All in-vivo spectra were processed with LC model [8] to calculate metabolite levels (Figure 1). Metabolites with <20% CRLB were incorporated for further analysis. Paired t-test was used to explore the difference among the control group at two time points (week 1 and week 5). One way ANOVA was used to test for group differences at three time points in CMS exposed animals.RESULTS
The preliminary diffusion MRI data analysis revealed significant microstructural alteration, such as D ̅ shows significant alteration in amygdala, W ̅ shows significant alteration in hippocampus and caudate putamen and W∥ showed significant alteration in prefrontal cortex. Magnetic resonance spectroscopy data (Figure 2) have shown significant increase in glutamine, N-acetyl aspartate (NAA) and combined ratio of NAA and N acetyl aspartyl glutamate (NAAG) on week 1 after CMS exposure. At week 8, all metabolite levels had no significant alteration in comparison to control, however significantly lower ratios of glutamine and combined glumatine and glutamate were observed in comparison to week 1.DISCUSSION
The preliminary diffusion tensor and diffusion kurtosis analysis reveals significant alteration in all the stress sensitive regions of the brain examined. The spectroscopy data at week 1 indicates activated ventral hippocampus which might be due to enhanced anxiety which is known to be present after CMS exposure [9, 10]. Unlike previous studies we observed significantly higher glutamine rather than glutamate. We find enhanced NAA and combined NAA and NAAG level in ventral hippocampus in agreement with previous reports [5]. This might indicate activated NAA metabolism in ventral hippocampus. Ex-vivo diffusion MRI data for neurite density estimation has been acquired and is being analysed. Quantitative histology will be performed on the brains as in [4] to further substantiate the present findings.CONCLUSION
We find significant microstructural alterations in hippocampus, prefrontal cortex, caudate putamen and amygdala in CMS induced depression in rats. MRS data also emphasizes the role of ventral hippocampus in CMS induced anxiety. This indicates a vital role of these brain regions in depression and that these effects can be interrogated with diffusion kurtosis imaging and MR spectroscopy techniques suitable for clinical use.Acknowledgements
Lundbeck Foundation R83-A7548 and Simon Fougner Hartmans Familiefond. Danish Ministry of Science, Technology and Innovation’s University Investment Grant (MINDLab, Grant no. 0601-01354B), and NIH 1R01EB012874-01, Lippert’s Foundation and Korning’s Foundation for financial support. Danish Research Council's Infrastructure program, the Velux Foundations, and the Department of Clinical Medicine, AU.References
1. McEwen, B.S. and P.J. Gianaros. Stress-and allostasis-induced brain plasticity. Annual review of medicine 2011; 62, 431.2. Wiborg, O. Chronic mild stress for modeling anhedonia. Cell and tissue research 2013; 354, 155-169.3. Willner, P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005; 52, 90-110.4. Khan, A.R., A. Chuhutin, O. Wiborg, et al. Biophysical modeling of high field diffusion MRI demonstrates micro-structural aberration in chronic mild stress rat brain. NeuroImage 2016.5. y Palacios, R.D., A. Campo, K. Henningsen, et al. Magnetic resonance imaging and spectroscopy reveal differential hippocampal changes in anhedonic and resilient subtypes of the chronic mild stress rat model. Biological psychiatry 2011; 70, 449-457.6. Hansen, B., N. Shemesh, and S.N. Jespersen. Fast imaging of mean, axial and radial diffusion kurtosis. NeuroImage 2016.7. Hansen, B., T.E. Lund, R. Sangill, et al. Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med 2013; 69, 1754-1760.8. Provencher, S.W. Automatic quantitation of localized in vivo1H spectra with LCModel. NMR in Biomedicine 2001; 14, 260-264.9. Bannerman, D., M. Grubb, R. Deacon, et al. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural brain research 2003; 139, 197-213.10. Jayatissa, M.N., C. Bisgaard, A. Tingström, et al. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 2006; 31, 2395-2404.Figures
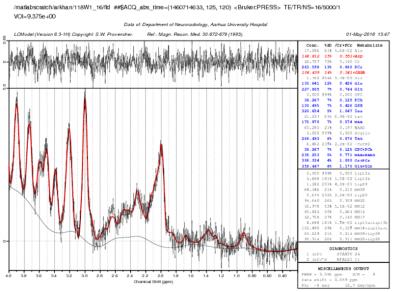
1H MR spectra from ventral hippocampus processed
on LC model.

Metabolite level normalized with tCr (Mean+S.D) in ventral hippocampus. Significant increase in Glutamine, NAA and NAA+ NAAG were observed only on week1 in comparison to control. Significantly lower glutamine and glutamine +glutamate metabolite ratio was observed in comparison to week 1. No significant difference were found between controls at two time points. (* p<0.05 : in comparison to control, # p<0.05: in comparison to week1).