1859
Effect of Velocity-compensated Diffusion Preparation for Spinal Cord Diffusion Imaging1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, People's Republic of China, 2Vascular Imaging Laboratory, Department of Radiology, University of Washington, Seattle, WA, United States
Synopsis
The spinal cord and surrounding cerebrospinal fluid undergo significant cardiac pulsations, which can influence the microscopic motion-sensitive diffusion preparation and cause signal void in the diffusion images. In this work, velocity-compensated diffusion-encoding gradient waveform was implemented in spinal cord diffusion imaging and the images are compared with traditional monopolar preparation and cardiac gating approaches. Results show that with velocity-compensated diffusion preparation, the spinal cord diffusion imaging shows fewer signal voids compared to the traditional monopolar diffusion preparation. Using velocity-compensated diffusion preparation without cardiac triggering can provide a new approach for spinal cord diffusion imaging.
Purpose
The spinal cord and surrounding cerebrospinal fluid (CSF) undergo significant cardiac pulsations, which can influence the microscopic motion-sensitive diffusion preparation and cause signal void in the diffusion images1. Thus cardiac gating is widely used to trigger single-shot EPI DWI scans in the window of relative mild motion, which can reduce the scan efficiency. Velocity-compensated (M1-compensated) diffusion preparation has been demonstrated to improve liver ADC measurement reproducibility without respiratory or cardiac triggering2. However, applying motion-compensated diffusion preparation for spinal cord diffusion imaging has been seldom reported. In this work, velocity-compensated diffusion-encoding gradient waveform was adopted in spinal cord diffusion imaging and the resultant images are compared with traditional monopolar preparation and cardiac gating approaches.Methods
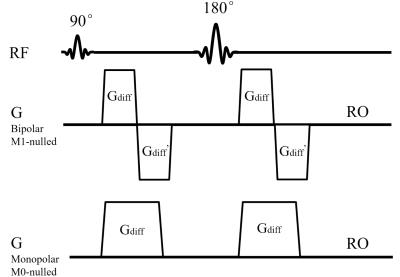
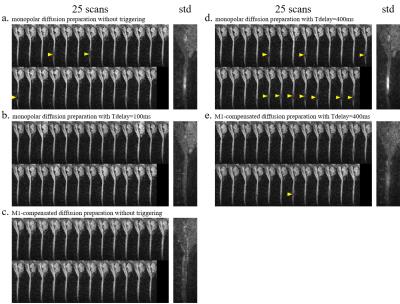
Human spinal cord DWI scans were performed on a Philips 3.0T MRI scanner (Philips Healthcare, Best, The Netherlands) using a 16-channel neurovascular coil. Data were acquired from one healthy volunteer under IRB approval from our institution. Finger pulse-oximeter signal was used for cardiac triggering. A cine scan was first performed to determine the trigger delays (Tdelays) for quiescent and pulsatile motion periods (Tdelay was set to 100 ms and 400 ms for quiescent and pulsatile motion respectively in the following scans). Bipolar diffusion-encoding gradient waveform was implemented with M1 nulled as in Fig. 1. For comparisons, traditional monopolar (Stejskal-Tanner) diffusion preparation and different Tdelays were also performed, in order to test the robustness of preparation under quiescent or pulsatile motion. Five scans were performed consecutively on the subject: a. monopolar diffusion preparation without triggering; b. monopolar diffusion preparation with Tdelay = 100 ms; c. M1-compensated diffusion preparation without triggering; d. monopolar diffusion preparation with Tdelay = 400 ms; e. M1-compensated diffusion preparation with Tdelay = 400 ms. Each scan was repeated 25 times for statistical analysis. The five scans used single-shot EPI sagittal acquisition with GRAPPA = 2, FOV = 220 × 110 mm2 with outer volume suppression (totally ZOOPPA = 2 × 2), acquisition voxel size = 2.53 mm3, TE = 76 ms, TR = 3000 ms (untriggered)/ 3 heart beats (triggered), diffusion preparation was applied in the SI directions with b = 600 s/mm2. The averaged images of the 25 repetitions were used to calculate the ADC maps.Results and Discussion
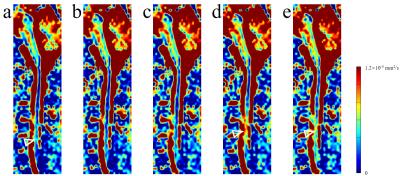
Fig. 2 shows spinal cord diffusion images of the 5 scans. For images using the traditional monopolar diffusion preparation, they show significant signal voids (pointed by yellow arrowheads) in the spine without triggering or in pulsatile motion periods (Tdelay = 400ms); but no obvious signal voids in the quiescent window (Tdelay = 100ms). Images using velocity-compensated diffusion preparation (M1-nulled) show fewer signal voids than with monopolar diffusion preparation in the same trigger window. The standard deviation of the 25 repetitions also demonstrates the stability of the velocity-compensated diffusion preparation modules. Fig. 3 shows the ADC maps of the 5 scans. Monopolar diffusion preparation at Tdelay = 100ms and velocity-compensated diffusion preparation without triggering show relative homogenous ADC along the spine, while other scans show higher ADC around C5 (pointed by hollow arrowheads), which can be resulted from the signal loss in the diffusion images.
Using velocity-compensated diffusion preparation without cardiac triggering, the ADC maps show comparable results to the conventional triggered approach. The spinal cord diffusion images using monopolar diffusion preparation without triggering may show some errors due to the CSF pulsation. With velocity-compensated diffusion preparation, the signal voids are reduced compared to the traditional diffusion preparation, even at the severe pulsation period (at Tdelay = 400ms in this experiment). Velocity-compensated diffusion preparation without cardiac triggering may provide a more efficient approach for spinal cord diffusion imaging, compared to the triggered methods. Prolonged TE (thus lower SNR) compared to monopolar diffusion preparation can be partially compensated by the CODE gradient design method3.
Conclusion
With velocity-compensated diffusion preparation, the spinal cord diffusion imaging shows fewer signal voids compared to the traditional monopolar diffusion preparation. Using velocity-compensated diffusion preparation without cardiac triggering can provide a new approach for spinal cord diffusion imaging.Acknowledgements
No acknowledgement found.References
1. Skare S, Andersson JLR. On the effects of gating in diffusion imaging of the brain using single shot EPI. Magn. Reson. Imaging 2001;19:1125–1128. doi: 10.1016/S0730-725X(01)00415-5.
2. Ozaki M, Inoue Y, Miyati T, Hata H, Mizukami S, Komi S, Matsunaga K, Woodhams R. Motion artifact reduction of diffusion-weighted MRI of the liver: Use of velocity-compensated diffusion gradients combined with tetrahedral gradients. J. Magn. Reson. Imaging 2013;37:172–178. doi: 10.1002/jmri.23796.
3. Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn. Reson. Med. 2016; doi: 10.1002/mrm.26166.
Figures