1858
Diffusion Tensor Imaging of human muscle at ultra-high-field (7T) MR.1Department of Biomedical Imaging and Image-guided Therapy- MR Centre of Excellence, Medical University of Vienna, Vienna, Austria, 2Orthopedic Department, Evangelisches Krankenhaus Wien, Vienna, Austria, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Ultra-high-field (7T) imaging already demonstrated to provide more robust DTI measurements in comparison to 1.5 and 3T in the brain but, to the best of our knowledge, it was not applied for DTI measurements on human muscles. Our results showed higher SNR as well as an overall improvement in DTI metrics for the entire calf muscle at 7T than at 3T. Single muscle analyses (gastrocnemii, tibialis anterior) demonstrated more heterogeneous results. Future studies including a larger population and considering technical challenges (e.g.,RF inhomogeneity, gradient performance) are necessary to assess if 7T may provide higher benefits in specific muscles.
PURPOSE
To compare Diffusion Tensor Imaging (DTI) measurements of human calf muscles at ultra-high-field strength (7T) with measurements at 3T.METHODS
Eight volunteers (5 males, mean age 30.25±3.65 years) without any history of muscle injury were examined at rest on a 7T Magnetom MR research system (Siemens Healthcare, Erlangen, Germany) and subsequently on a 3T MAGNETOM Trio MR, a Tim System (Siemens Healthcare, Erlangen, Germany) using a 28-channel and an 8-channel knee coil, respectively. STEAM-based DTI was applied using a prototype ss-EPI sequence with diffusion time 200ms, GRAPPA-2, FatSat, b-values 0 and 500s/mm2, 6 averages, 12 directions; 30 adjacent axial slices of 3.5mm thickness. To correct frequently occurring artifacts (i.e., areas of signal loss) affecting the STEAM-based DTI images, a previously tested method based on the weighted mean of voxels’ signal intensity was applied [1]. DTI is sensitive to signal-to-noise ratio (SNR) and because ultra-high-field strength MR as much as STEAM sequence are expected to enable high SNR, thus SNR at 3T and 7T was calculated and compared. The SNR was computed as the ratio of the signal amplitude of the muscles (i.e, mean of the signal amplitude inside two ROIs placed respectively in the central and peripheral muscles of the calves) to the background noise measured outside the calf [2]. To investigate only muscle tissue, a mask was automatically obtained for each calf, using histograms of signal intensities and multiplying the correspondent MD and RD maps (Matlab). Forth-order Runge-Kutta (RK4) tracking was applied (DSI Studio Software, http://dsi-studio.labsolver.org) and different FA and angle threshold were used: 0.12 and 0.17° [1] for the entire calf, 0.15 and 20° for GM and GL [3] and 0.15 and 0.45° for TA [4]. Entire calf as well as single muscle DTI measurements (i.e, track number, length, and volume, fractional anisotropy (FA), mean- (MD), axial (AD) and radial diffusivity (RD)) obtained from freehand ROI of the tibialis anterior (TA), gastrocnemius medialis (GM) and lateralis (GL) were performed (DSI Studio). SNR as well as DTI metrics measurements derived from 7T and 3T DTI datasets were then compared using Student’s t-tests.RESULTS
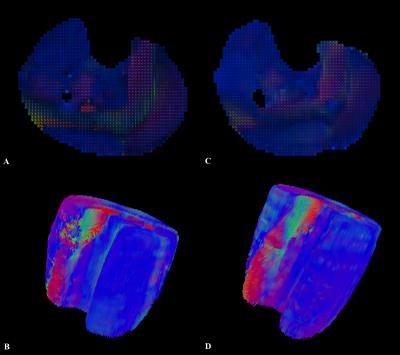
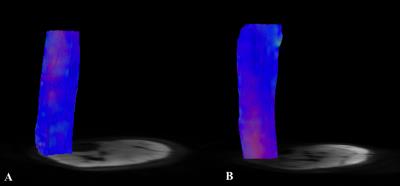
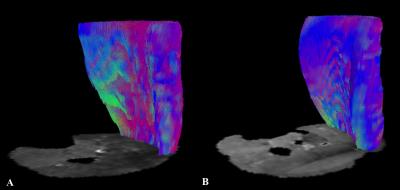
All volunteers were successfully investigated at 7T and 3T. The SNR was significantly higher (+12.33%) at 7T than at 3T (mean values±SD, 56.04±7.22 and 49.89±6.39, respectively; p=0.042). DTI measurements of the entire calf demonstrated significantly higher FA, AD, number, length as well as greater volume of muscle fibers (+11.70%, +6.23%, +136.22%, +35.94% and +25.87%, respectively; p<0.05 each) at 7T. There were no significant differences for MD and RD (p>0.05,each) (Fig. 1). At 7T, both GM and GL showed longer fibers (+37.36%, +51.36%) with greater volume (+14.39%, +48.65%); GM demonstrated also higher AD (+2.31%) and GL showed higher FA (+6.11%) (p<0.05, each). Significantly longer fibers (+16.98%) as well as higher FA (+13.46%), MD (+14.99%), AD (+19.40%) and RD (+11.14%) were measured for TA at 7T (p<0.05, each) (Fig. 2 and Fig. 3).DISCUSSION
Several studies already investigated the feasibility of DTI in the brain at ultra high field showing higher SNR and more robust DTI metrics even if challenges are still associated with some areas as, for instance, the temporal lobe [5,6]. Up to now, to the best of our knowledge, DTI was not applied at 7T for investigating muscles and our results demonstrated that in the overall calf muscle, for all DTI metrics, but MD and RD, a significant improvement occur at ultra high field. As already observed in the brain, also in the muscle, heterogeneous results emerged examining different areas. Improvement in length and volume of the tracks occurred in the gastrocnemii and higher FA in the GL and TA, but in the latter muscle these were associated also with increased MD values. The dependence of these findings to technical challenges as high magnetic susceptibility at 7T is expected to be further assessed.CONCLUSIONS
Ultra high field demonstrated higher SNR and an overall improvement in DTI measurements of the calf muscle in comparison to 3T. Future studies including a larger cohort and considering technical challenges as RF inhomogeneity and gradient performance are necessary to further characterize the advantages in single muscle analyses and evaluate if 7T could provide greater benefits especially in specific muscles.Acknowledgements
No acknowledgement found.References
1. Giraudo C, Motyka S, Trattnig S, Bogner W. Towards robust Diffusion Tensor Imaging of skeletal muscles via an automatic artifact removal tool. ISMRM 24th Annual Meeting, 2016, Singapore.
2. Kaufman L, Kramer DM, Crooks LE, Ortendhal DA. Measuring Signal-to-noise ratios in MR imaging. Radiology 1989;173:265-267.
3. Sinha U, Csapo R, Malis V, et al. Age-Related Differences in Diffusion Tensor Indices and Fiber Architecture in the Medial and Lateral Gastrocnemius. JRMI 2015 41:941-953.
4. Heemskerk A, Sinha TK, Wilson KJ, et al. Quantitative Assessment of DTI-Based Muscle Fiber Tracking and Optimal Tracking Parameters. Magn Res Medic 2009 61:467-472.
5. Polders DL, Leemans A, Hendrikse J, et al. Signal to Noise Ratio and Uncertainty in Diffusion Tensor Imaging at 1.5, 3.0, and 7.0 Tesla. JMRI 2011; 33:1456-1463.
6. Wiggins CJ, Benner T, Wiggins GC, et al. In-vivo, Human Diffusion Tensor Imaging at 7T: First Results. ISMRM 2007.
Figures