1855
Robustness of kurtosis acquisition via simultaneous multi-slice EPI: a test-retest in children1Medical Physics department, Bambino Gesù children's hospital, Rome, Italy, 2Imaging Department, Bambino Gesù children's hospital, Rome, Italy, 3Medical Physics department, Bambino Gesù children's hospital, ROME, Italy
Synopsis
Diffusion kurtosis imaging is an emerging technique based on non-gaussian diffusion of water in biologic systems and provides complementary information to the traditional diffusion. Although the method is very promising in identifying new biomarkers, it suffers from long time acquisition, which is very challenging in . However, a recent technique, named simultaneous multi-slice (SMS) acquisition, allows multiple slices acquisition thus drastically reducing the acquisition time. The purpose of this work is then to study the robustness of diffusion kurtosis in children when acquired via sms method.
Introduction
Diffusion kurtosis imaging is an emerging technique based on non-gaussian diffusion of water in biologic systems. The method provides complementary information to the traditional diffusion and its application to brain tumour to identify novel biomarkers has been considered very promising1. Unfortunately, diffusion kurtosis suffers from long time acquisition, which tends to reduce the applicability to clinical environment. This issue is even more pronounced when the kurtosis data are acquired in children because of either scarce compliance or the use of general anesthesia. The first might cause motion artifacts whereas for the latter it would be beneficial for the patient to keep the scanning time as short as possible. However, a recent technique, named simultaneous multi-slice (SMS) acquisition2, allows multiple slices acquisition thus drastically reducing the acquisition time. Since the drawback of this method is lower Signal-to –Noise ratio and kurtosis acquisition implies the use of high b value, it is of extreme importance to investigate the reproducibility of the measurements and determine its variability. The purpose of this work is then to study the robustness of diffusion kurtosis in children when acquired via sms method.Materials and Methods
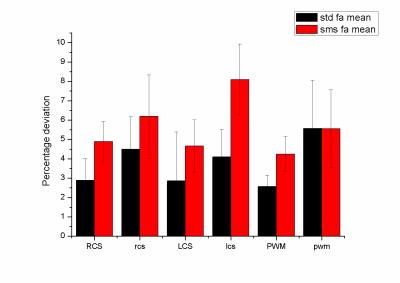
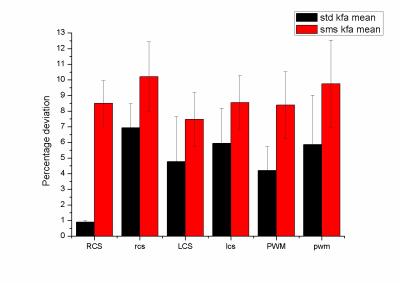
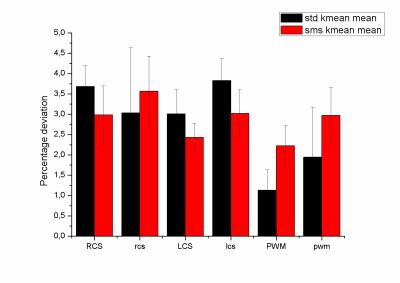
Sixteen (age range of 6-10 years) children undergoing to standard clinical MRI protocol were selected. The selection criteria applied were based upon no evidence of diffusive white matter abnormalities. Two diffusion weighted acquisitions at the begging and the end of scanning were acquired. A 3T MAGNETOM Skyra (Siemens Healthcare, Erlangen, Germany) equipped with a 20-channel head/neck coil was used. For 6 out of the 16 patients the diffusion protocol used was the standard acquisition (total acquisition time=10 minutes; FOV = 256x256mm, 46 slices, TR/TE = 9000/114ms, BW = 1562Hz/px, matrix resolution = 128x128, slice thickness = 2.0mm, GRAPPA factor = 2, 30 directions, b values=0,1000,2000 s/mm2;) ; for 10 out of 16 patients the diffusion protocol used was the multi-slice (SMS) acquisition (total acquisition time=5 minutes; FOV = 256x256mm, 46 slices, TR/TE = 4600/113ms, BW = 1562Hz/px, matrix resolution = 128x128, slice thickness = 2.0mm, GRAPPA factor = 2, Slice acceleration factor =2, Verse factor=2.2, 30 directions, b values=0,1000,2000 s/mm2) which was acquired using a prototyping sequence from Siemens. Both set of diffusion images were preprocessed in matlab to account for eddy current and moving artifact by using ACID toolbox3 for spm. The actual kurtosis processing was performed on DKE toolbox. FA, kFA, kmean maps were calculated. Two circular ROI sizes were used, small (diameter = 4 mm) and large (diameter = 8 mm) identifying them with small or capital letters. ROIs were placed by an expert neuroradiologist in three brain areas: right (RSC, rsc) and left(LSC, lsc) semiovale centrum white matter, and posterior white matter(PWM, pwm). The absolute difference (AD) between the first and last scan scaled by the mean was used to evaluate the variability of the measure. T-tests between the two acquisition protocols were performed.Results
The figure 1,2 and 3 show the differences between the standard and the multi-slice protocols for mean FA, KFA and Kmean values within the ROis. There was significant difference between the protocols (p<0.05) in FA and kFA but not in Kmean. The varialbility for FA and KFA was around 40% smaller in the standard protocol compared to sms one. Smaller ROis have larger variability than the large ones.Discussion
To the best of our knowledge this is the first study investigating the variability of diffusion parameters acquired with multislice and standard sequence diffusion. In particular, although the kmean variability seems not to be affected by the sequence, the FA and KFA maps show smaller variability probably due to lower SNR.Conclusion
Even though the study indicates how the use of diffusion kurtosis acquired via multi-slice acquisition in children leads to less stable measure compared to standard procedure, the observed variability is always below 10%. This evaluation is important in order to decide about the number of pediatric subjects to be included in a kurtosis study as well as to consider to exploit the use of coils with larger number of channels to enhance the SNR.Acknowledgements
We would like to thank dr Beck Thomas from Siemens for the useful comments and for providing the prototyping sequenceReferences
1) Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed 2010; 23:836–848
2) Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001;13:313–317.
2. Mohammadi S, Moller HE, Kugel H, Muller DK, Deppe M. Correcting eddy current and motion effects by affine whole-brain registrations: evaluation of three-dimensional distortions and comparison with slicewise correction. Magn Reson Med 2010;64: 1047-1056.
Figures