1838
Joint estimation of free water and perfusion fraction in human brain1Department of Medical Radiation Physics, Lund University, Lund, Sweden, 2Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 3Department of Radiology (Adjunct), Johns Hopkins School of Medicine, Baltimore, MD, United States
Synopsis
The perfusion of blood affects the estimation of diffusivities, especially fast components such as free water. Here, we acquired human data to demonstrate the applicability of a three-compartment model for the joint estimation of tissue diffusivities, free water, and the perfusion fraction. We evaluated the feasibility of the model by comparing a multiple b-value approach with a shorter, clinically feasible approach. The conclusion is that the two-compartment free-water estimation is affected by both water and blood. The three-compartment model disentangles these effects, useful in distinguishing between changes originating from capillary blood from those originating from the extracellular space.
Introduction
The free-water fraction provides meaningful contrast in various clinical1,2,3,4,5 as well as normal development and aging studies6. In a typical diffusion MRI experiment, free water, i.e., water molecules that exhibit free diffusion, is found in extracellular spaces. However, a typical volume element may also include other pools of fast diffusing processes, such as the water molecules in perfusing blood in the capillary network. Simulations have shown that this Intravoxel-Incoherent-Motion-Imaging (IVIM) effect causes overestimation of free water, which can be resolved considering a three-compartment model7. We demonstrate the feasibility of applying the three-compartment model to human data, acquired with dense b-shells as well as with a short, clinically feasible protocol.Methods
A comprehensive diffusion MRI measurement was performed using a non-product spin-echo EPI sequence on a 3T Siemens Prisma with diffusion encoding in six directions, with 44 b-values ranging from 0 to 800s/mm2 (TR=4500ms, TE=67ms, 1.3mm×1.3mm×4mm, 32 slices). A clinical diffusion sequence was acquired on a 1.5T Siemens Avanto with b-values of 0, 50, 200, 500, 900 and 1400s/mm2 (TR=8500ms, TE=85ms, 2.5mm×2.5mm×2.5mm, 56 slices). The model below was fitted (non-linear least squares) to data from both acquisitions:
$$ S_i=S_0 ⋅[f_b⋅e^{-b_i D^* }+f_w⋅e^{-b_i D_w}+(1-f_b-f_w )⋅e^{-b_i g_i^T D_t g_i} ]$$
for gradient direction gi, with b-value bi. The pseudo-diffusion coefficient D* was set to 10-2mm2/s and free-water diffusivity Dw was set to 3×10-3mm2/s. The free parameters were a positive definite cylindrical symmetric tensor, Dt, and the positive fractional volumes fb<1 and fw<1 of the blood and water compartments, respectively. The two-compartment fit implied that fb=0, and the one compartment fit implied that fw=0. FA and MD were calculated from Dt for each model fit. All parameters were registered to MNI space and averaged over white matter regions of interest (ROIs) defined by the JHU DTI-based atlas. To further demonstrate the ubiquity of the IVIM effect, we simulated a conventional multi-shell acquisition without low b-values (but including b=0). Simulations included three-compartment signal (blood, free water and tissue, both anisotropic and isotropic) for different blood fractions.
Results
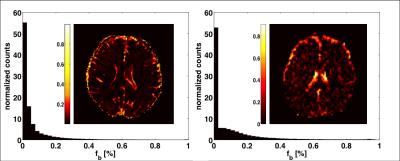
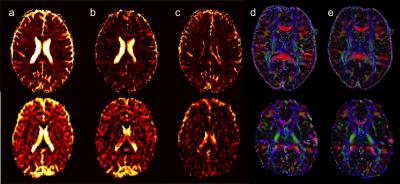
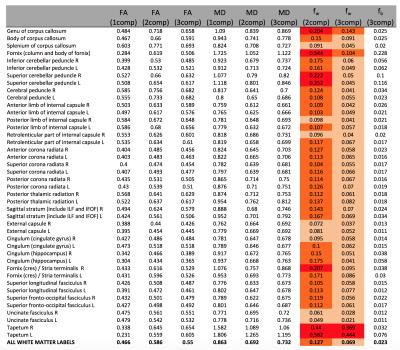
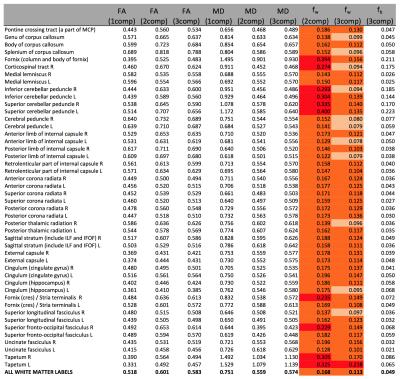
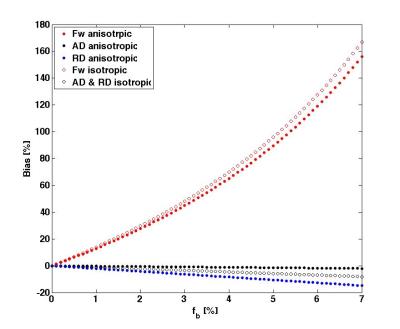
Figure1 shows the distribution of fb for the two acquisition types. Values were ranging between 0 and 10%. Representative slices of fw and FA maps are shown in Figure2. Figure3 shows that the whole brain average of fb in the comprehensive acquisition was 2.4% (equivalent to ~50% overestimation of fw)7. The mean fw decreased from 12.7% for the two-compartment model to 6.9% for the three-compartment model. Figure4 shows that for the clinical acquisition, the whole brain average of fb was 1.2%, and the mean fw decreased from 16.8% to 11.3%. For the white matter ROIs in both acquisitions, differences in FA were most prominent between the one- and two-compartment models, and less prominent when comparing the two- and three-compartment models. The largest effect between the two-and three-compartment models was for the overestimation of fw. Figure5 demonstrates the effect of IVIM using b=0 and b>500. As long as b=0 is included, there is still a bias in the estimated parameters.Discussion
Our analyses present comparable results between the comprehensive and clinical acquisitions, demonstrating the feasibility of the three-compartment model. As more low b-values are included, a higher fb is estimated, suggesting underestimation of fb with the clinical sequence. Nevertheless, we show that the IVIM effect persists even when including a single b=0 without additional low b-values. Following IVIM correction, fw is reduced to values below 10%, which is more in line with expected fractional volumes of extracellular spaces in healthy brain. The reduction in fw is especially prominent in the Fornix, which may explain previous findings of higher than expected fw values in this region with the two-compartment model8. On the other hand, estimation of FA appears to be less affected by IVIM, although it is affected if free water is neglected, suggesting that two-compartment model estimations of FA are valid. The suggested three-compartment model overcomes the IVIM effect, and in addition, provides potentially useful contrast patterns based on separation of the volume fraction of blood from the fractions of free water and tissue.Conclusion
A three-compartment model is important to disentangle the IVIM effect from that of free water, distinguishing between changes that originate from capillary blood flow and those that originate from the extracellular space. The model estimation can be improved by acquiring low b-values, as well as model fitting approaches. Separating these effects allows for a more specific characterization of heterogeneous tissues, which is especially important in disorders involving a combination of vascular, edematous, and tissue changes such as Alzheimer’s disease, vascular dementia, and brain injuries.Acknowledgements
We acknowledge Siemens for granting access to product sequence source code.References
References: [1] Pasternak O et al. J Neurosci (2012), [2] Maier-Hein KH et al. Alzheimers Dement (2015), [3] Ofori E et al. Brain (2015), [4] Pasternak O et al. J Neurosci (2014), [5] Pasternak O et al., Schizophrenia research (2016), [6] Metzler-Baddeley C et al. Neuroimage (2012), [7] S. Rydhög A, ISMRM Workshop on Breaking the Barriers of Diffusion MRI (2016), [8] De Santis S et al. Neuroimage (2014)Figures