1837
Towards a practical protocol for accurate and reliable MR-derived diffusion changes of the optic nerve in optic neuritis1Diagnostic Radiology, Singapore General Hospital, Singapore, Singapore
Synopsis
Optic neuritis is a demyelinating inflammation of the optic nerve that often occurs in association with multiple sclerosis and neuromyelitis optica. Magnetic resonance imaging (MRI), especially diffusion-tensor imaging (DTI) is highly sensitive for inflammatory changes in the optic nerves. We propose a DTI protocol which balances susceptibility artefacts, scan time, and resolution for better image quality and clinical practicality. It allows a 1 mm in-plane resolution in order to avoid partial volume averaging with cerebrospinal fluid (CSF) surrounding the optic nerve in examining fractional anisotropy (FA) values and apparent diffusion coefficient (ADC) for severity of optic neuritis.
Purpose
Our specific aims were i) to establish a practical protocol for evaluation of the optic nerves by DTI and ii) to evaluate differences in diffusion quantities between the optic nerves of clinically diagnosed optic neuritis1 patients, and those of normal controls.
Methodology
We propose three steps towards a practicable protocol, viz:
1) Measuring the diffusion weighted signal of the intraorbital optic nerve is an imaging challenge with regard to distortion artefacts. They arise from the nerves’ small size and huge susceptibility differences between nearby bony structures, lipids, air-filled paranasal sinuses and surrounding CSF. Reduced susceptibility artefacts are achieved by means of read-out segmented echo-planar imaging (EPI) with a corresponding reduction in EPI echo spacing; abbreviated as RESOLVE2.
2) Is diffusion weighted imaging (DWI) or DTI the method of choice ? DWI provides diffusion information along three directions within the Cartesian coordinate system (frequency-encoded, phase and slice-select directions), which does not allow a comparison of left and right optic nerve within the same scan.
The advantage of DTI (over DWI) is the sampling of relative diffusion coefficients in unique encoding directions, with a minimum number of six. As such, DTI provides quantities irrespective of the position of the optic nerve within the gradient system, namely fractional anisotropy (FA), trace ADC, axial and radial diffusivity.
3) Is the scan time longer for DTI compared to DWI ? High resolution DWI of the optic nerve requires signal averaging for adequate signal-to-noise ratio (SNR), which is comparable in time to the acquisition of a larger number of diffusion encoding directions in DTI.
The trade-off between SNR, resolution, and imaging time led to the parameters for a diffusion-weighted echo-planar imaging (EPI) sequence: RESOLVE factor 13: TR/TE 1200ms/62ms; 30 directions; b values 0(x3), 800 s/mm2; FOV 170x170; matrix 168x168; slice thickness 3.5 and total imaging time, 7 minute 30 seconds. The study was performed on a 3T scanner together with a 32-channel head coil (Magnetom Skyra, Siemens Heathcare Sector, Erlangen, Germany).
Controls and Patients
Institutional review board (IRB) approval was obtained. Fifteen volunteers were enrolled in the study. Regions of interest (ROIs) of 6-9 mm2 were drawn in the anterior and posterior parts of bilateral intraorbital nerves on axial sections. Fourteen patients presented clinically with optic neuritis and T2WI and post-contrast T1WI confirmed area of inflammation in the intraorbital segment of the optic nerve. Two patients with inflammation of the chiasm but normal appearing intraorbital optic nerves were excluded.
Results
The shorter EPI echo spacing of RESOLVE results in a reduction in the level of geometric distortion and susceptibility artefact (Figure 1).
The normative diffusion quantities are categorized according to the two quantities under investigation:
1) The mean±SD value of FA in the anterior (0.50±0.07) and posterior (0.53±0.08) part of the intraorbital segment in controls is shown as a solid line (Figure 2a);
2) ADC in the anterior (980±180x10-6mm2/s) and posterior (930±125x10-6mm2/s) part in Figure 2b.
Patient data are indicated with symbols.
Discussion
Although other authors3 claim to have measured diffusion parameters of the optic nerve based on single-shot DWI-EPI, we were not convinced that we could delineate the intraorbital segment of the optic nerve in its full length. We therefore preferred read-out segmented EPI at the cost of acquisition time. Furthermore, the trade-off between resolution and risk of contamination with CSF in the subarachnoid space around the optic nerve lead to an additional acquisition time due to the high number of directions (namely 30) for adequate SNR. To minimize arbitrary eye-movements, volunteers and patients were asked to gaze at a round low contrast shape.
In the control group, the posterior part of the intraorbital segment of the optic nerve shows lower FA compared to the anterior part, which is in line with the brain-to-eye direction of myelination4. For ADC, it is the reverse. In the patient group, areas of abnormal contrast enhancement showed either ‘preserved’ FA together with low ADC (triangles) or decreased FA together with high ADC (diamonds), possibly due to different time periods after onset of optic neuritis. Similar to multiple sclerosis5, it may reflect a conversion from restricted diffusion in the acute phase to vasogenic edema in the subacute phase of optic neuritis (Figure 2). In a larger patient group, DTI based on this protocol may be able to assess microstructural changes and damage of the optic nerve in a more graded manner.
Conclusion
RESOLVE allowed DTI imaging of the entire intraorbital segment of the optic nerve with significantly reduced distortions. Our findings suggest that diffusion quantities may be useful for further evaluating the pathophysiology of acute optic neuritis.
Acknowledgements
This work was supported by the SingHealth Duke-NUS Radiological Sciences Academic Clinical Programme.
References
1 Balcer LJ. Clinical Practice. Optic Neuritis. N Engl J Med. 2006;354:1273-1280
2 Porter DA, Heidemann RM. High resolution diffusion weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62(2):468–475
3 Wang MY, Qi PH, Shi PD et al. Diffusion Tensor Imaging of the Optic Nerve in Subacute Anterior Ischemic Optic Neuropathy at 3T. AJNR 2011;32(7):1188-94
4 Goldberg JL. Optic Nerve. Editors: Kaufman PL, Adler FH, Leonard A, Levin LA et al. Adler’s Physiology of the Eye, 11th edition, 2011:550-574
5 Balashov KE, Aung LL, Dhib-Jalbut S, Keller IA. Acute Multiple Sclerosis Lesion: Conversion of Restricted Diffusion Due to Vasogenic Edema. J Neuroimage 2011;21(2):202–204
Figures
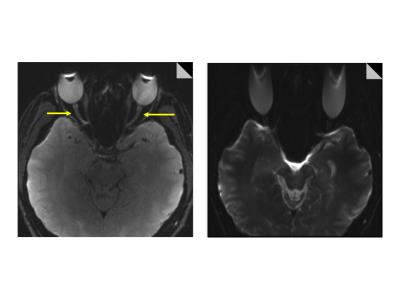
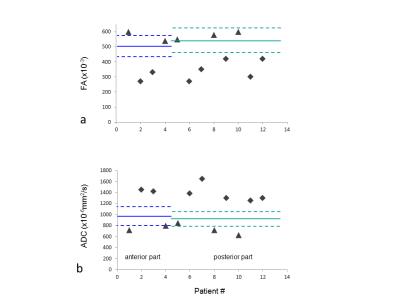
Figure 2: Control diffusion quantities, (a) FA and (b) ADC, are shown as mean value (solid line) and 1 SD (dashed lines) for the anterior and posterior part of the intraorbital segment of the optic nerve. Individual patient data are shown as symbols.
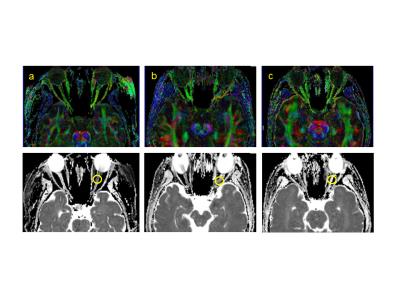
Figure 3: Representative case illustration with FA map (top) and ADC map (bottom). a) 21-year-old female presented with left neuro-myelitis optica. ADC suggests cytotoxic edema. b) 40-year-old female presented with left optic neuritis. ADC is pseudo-normalized.c) 41-year-old female with a history of recurrent optic neuritis demonstrating vasogenic edema.