1835
Fiber connection density differences detected in patients with sickle cell disease1Radiology, Children's hospital Los Angeles, Los Angeles, CA, United States, 2Radiology, Children's hospital, Los Angeles, CA, United States, 3Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 4Neuroscience Graduate Program, University of Southern California, Los Angeles, CA, United States, 5Sherbrooke Connectivity Imaging Lab (SCIL), Computer Science Department, University of Sherbrooke, Sherbrooke, QC J1K 0A5, Canada, 6Hematology, Children's hospital, Los Angeles, CA, United States, 7Cardiology, Children's hospital, Los Angeles, CA, United States
Synopsis
Sickle cell disease (SCD) is a chronic disorder characterize by progressive cerebrovascular damage. We hypothesized that subtle cerebral injury might be visible with diffusion imaging data in these patients. Tractography based on the fiber orientation distribution function (ODF) was applied in order to investigate the character and severity of white matter injury in patients with SCD. We found both decreased and increased fiber density in patients, compared to control subjects that co-localized with silent cerebral infarctions. These data suggest progressive white matter injury and compensatory mechanisms in SCD patients.
Introduction
Sickle cell disease (SCD) is a genetic disorder caused by a mutation in the gene encoding for the beta subunit of hemoglobin and is associated with anemia, chronic systemic and cerebral vasculopathy and strokes. Recent large studies of neurologically asymptomatic children and adults with SCD have demonstrated that cognitive impairment occurs even in absence of brain abnormalities on conventional magnetic resonance imaging. This suggests brain injury in SCD is diffuse and occurs at a microstructural level invisible to standard imaging. Evaluating white matter microstructure may provide better insights into the neurocognitive deficits observed in SCD patients. Recent studies probing WM microstructure have primarily relied upon a diffusion tensor model1-3. For SCD patients, decreased fractional anisotropy was found in major brain white matter tracts, such as the corpus callosum, the cortico-spinal tract and inferior fronto-occipital fasciculus, indicating decreased WM fiber integrity, suggesting insufficient oxygen delivery to neuronal tissue. White matter fiber tractography is most commonly implemented using the principal diffusion direction of the diffusion tensor. However, based on Gaussian diffusion assumption, this model implies that there can only be a single fiber population per voxel. High angular diffusion imaging techniques was utilized to better resolve crossing fibers. In this abstract, we perform tractography based on the fiber orientation distribution function (ODF) to investigate WM injury in patients with SCD. WM fiber density maps were created to assess whole brain connectivity and to act as surrogates for neurovascular injury.Methods
This study was approved by the institutional review board and performed at Children's Hospital Los Angeles. We recruited 15 SCD patients and 13 ethnically matched control subjects. Exclusion criteria were: previous stroke, current pregnancy and acute chest pain that required hospitalization within one month. All participants underwent a battery of imaging: structural and diffusion imaging and neurophysiological tests. Diffusion imaging scans were obtained in 30 directions using an EPI sequence with a b-value of 1000s/mm2. 3D T1 and T2 images were collected for volumetric assessments and detection of white matter strokes. The DTI data were processed, using the Diffusion Imaging in Python (DiPy4). Fiber ODFs of order 6 were estimated using constrained spherical deconvolution5. All the diffusion images underwent skull stripping, distortion correction, followed by Rician adapted non-local means filtering6. We used the fiber tracking algorithm proposed in 7. A density map of neural fiber tracts was performed for each subject. To compare these maps of SCD patients to that observed in control subjects, a two-sample Student test was used combined with a Monte Carlo simulation.Results
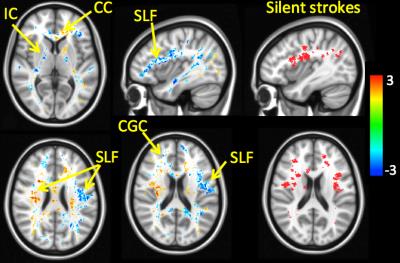
Right hand figures demonstrate the cumulative white matter stroke map across all patients as a binary mask. As displayed on Figure 1, we revealed not only areas with decreased but also increased fiber density in patients compared to control subjects. Decreases of fiber density were detected in the right anterior corpus callosum (CC), left anterior superior longitudinal fasciculus (SLF), right anterior internal capsule (IC), left posterior IC, right cingulum (CGC), bilateral inferior longitudinal fasciculus (ILF) and left inferior occipitofrontal fasciculus. In the frontal lobe, the distribution of reduced fiber density colocalized with regions of the white matter stroke map. The areas with higher fiber density in patients were located in the left anterior corpus callosum, right superior longitudinal fasciculus, left anterior internal capsule, left cingulum and bilateral corticospinal tracts.Discussion
Our diffusion imaging data showed widespread systematic decreased fiber density in patients with SCD in regions at risk for stroke. This suggests white matter damage is occurring, even in patients who don’t have overt strokes. Interestingly, regions of increased fiber density were also found adjacent to regions of high silent stroke probability. These may represent a compensatory mechanism for the frontoparietal damage and may explain the relatively mild neurocognitive deficits observed in our SCD group.Acknowledgements
This work was supported by the National Heart Lung and Blood Institute U01HL117718.References
1. Scantlebury N, Mabbott D, Janzen L, Rockel C, Widjaja E, Jones G, et al. White matter integrity and core cognitive function in children diagnosed with sickle cell disease. Journal of pediatric hematology/oncology. 2011;33(3):163-71.
2. Sun B, Brown R, Hayes L, Burns T, Huamani J, Bearden D, et al. White matter damage in asymptomatic patients with sickle cell anemia: screening with diffusion tensor imaging. American Journal of Neuroradiology. 2012;33(11):2043-9.
3. Chai Y, Coloigner J, Qu X, Choi S, Bush A, Borzage M, et al. Tract specific analysis in patients with sickle cell disease. 11th International Symposium on Medical Information Processing and Analysis (SIPAIM 2015); 2015: International Society for Optics and Photonics. 2015; p. 968108--6.
4. Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, Nimmo-Smith I and Dipy Contributors. Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics. 2014; vol.8, no.8.
5. Descoteaux M, Deriche R, Knosche TR, Anwander A. Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE transactions on medical imaging. 2009;28(2):269-86.
6. Descoteaux, M., N. Wiest-Daesslé, S. Prima, C. Barillot, and R. Deriche. Impact of Rician Adapted Non-Local Means Filtering on HARDI. Medical Image Computing and Computer-Assisted Intervention (MICCAI). 2008; 122-130, 2008.
7. Girard, G., Whittingstall, K., Deriche, R., & Descoteaux, M. Towards quantitative connectivity analysis: reducing tractography biases. Neuroimage. 2014, 98, 266-278.