1822
Rectal Adenocarcinoma: Diagnostic Accuracy of Diffusion Kurtosis Imaging (DKI) and its Correlation with Prognostic Factors1Radiology, Ruijin Hosptial, Jiaotong University, Shanghai, People's Republic of China, 2MR Collaboration NE Asia, Siemens Healthcare, Shanghai, People's Republic of China, 3APPL, Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, People's Republic of China
Synopsis
The high resolution MRI and conventional DWI suffers from unsatisfying accuracy in assessment of some prognostic factors of rectal adenocarcinoma. We employed DKI, a protocol mainly for evaluating the complexity of biologic tissues, to explore its diagnostic accuracy and correlation with prognostic factors. The kurtosis of DKI exhibited highest correlation with histologic grades especially the new grading criterion and higher potential in differentiation of high- and low grade tumors, and also in predicting nodal status, with higher specificity than or equivalent sensitivity to diffusivity and ADC. In conclusion, DKI could be a promising tool in predicting prognosis of rectal adenocarcinoma.
Introduction
High resolution magnetic resonance imaging and diffusion imaging has been widely employed as an essential imaging tool for accurate preoperative assessment for selecting high risk patients who could benefit from the more aggressive multimodality treatment. However, some of prognostic factors have to be confirmed through histopathological examination of surgical specimens. This study aimed to assess whether DKI-derived parameters of patients with rectal adenocarcinoma could provide more preoperative information correlated with prognostic factors. The prognostic factors are defined by both conventional WHO grading system and the new PDC grading system, which has been proven to be more reproducible and prognosis-related.Methods
56 parents (15 women, 41men; median age 60 years) diagnosed with primary rectal adenocarcinoma by endoscopic biopsies underwent preoperative MRI using a 1.5T MR scanner (MAGNETOM Aera, Siemens, Erlangen, Germany) with a dedicated six-channel body coil between April 2014 and September 2015. The written informed consent had been achieved from all patients. MRI sequences consisted of T2-weighted turbo spin-echo sequence without fat saturation in sagittal, oblique axial and oblique coronal directions and conventional DWI (b = 0, 1000 sec/mm2). Then DKI (b= 0, 700, 1400 and 2100 sec/mm2 sequence was added, and Kurtosis and diffusion coefficients from DKI and apparent diffusion coefficient (ADC) from conventional DWI were calculated by using an in-house developed software based on MATLAB (Mathworks, Natick, Mass). ROI-based mean values of 3 parameters were measured by two radiologists separately. Student t test, receiver operating characteristic curves, and Spearman correlation were used for statistical analysis.Results
Kurtosis of high-grade rectal adenocarcinomas was significantly higher than that of low-grade according to PDC grades (1.136±0.086 vs 0.988±0.060, respectively; p<0.05) and WHO grades (1.103±0.086 vs 1.034±0.103, respectively; p<0.05). In PDC grading, the diffusivity and ADC were significantly lower in high-grade tumors than in low-grade tumors (1.187±0.150 * 10-3 *mm2/sec vs 1.306±0.129*10-3*mm2/sec and 1.020±0.113*10-3*mm2/sec vs 1.108±0.097 *10-3*mm2/sec, respectively; p<0.05) and showed similar correlations with histologic grades (r=-0.486 and r=-0.406, respectively; p>0.05). Compared to both diffusivity and ADC, kurtosis showed significantly higher sensitivity (83.3% vs 70.8% and 70.8%, respectively) and specificity (96.8% vs 84.4% and 81.3%, respectively). Kurtosis showed a better correlation with PDC grades than with WHO grades (r=0.797 vs r=0.293, respectively; p<0.05). Kurtosis was significant higher in pN1-2 than in pN0 tumors (1.086±0.103 vs 1.009±0.086, respectively; p<0.05). All parameters manifest no significant differences in T stages, and no significant differences between low- and high-grade tumors were found in diffusivity and ADC.Discussion & Conclusion
The histologic grade is a stage-independent prognostic factor in rectal adenocarcinoma(2). Kurtosis in high-grade tumors was significantly higher than in low-grade tumors and showed a significant though weak correlation with WHO grades in our study. As we know, microstructures of high-grade tumors have higher complexity than that of low-grade tumors based on the WHO grading criteria. Namely, water motion in high-WHO-grade tumors may be more restricted, resulting in more non-Gaussian diffusion. The inhomogeneity caused by this difference might be the main reason for the greater kurtosis in high-WHO-grade tumors in our study. Also, kurtosis in high-PDC-grade tumors was significantly higher than in low-PDC-grade tumors. Tumors in high PDC grades have more poorly differentiated clusters, resulting in a greater restriction of water motion and then higher kurtosis. In addition, a significant correlation was found between kurtosis and PDC grades. There was an “abnormal” (extreme) point in the G3 subgroup using the PDC grading system as shown in the Results, which was classified as G2 according to the WHO grading criteria. A more marked inflammatory reaction, indicating a greater decrease in hindered and restricted water in the tumor, which resulted in less non-Gaussian properties, was observed. Therefore, the kurtosis of the lesion was smaller than that of other G3 rectal adenocarcinomas. We also found only the kurtosis was significantly higher in the pN1-2 group than in the pN0 group, suggesting that it may potentially predict patients with nodal involvement, better than ADC(3). In conclusion, Kurtosis and diffusivity from DKI model could serve as important prognostic parameters to aid therapy selection in patients with rectal adenocarcinoma especially kurtosis.Acknowledgements
We hold the honor to acquire support from the fund of Key Project of Science and Technology of Shanghai (Grant No. 16511101101) ,Major Program of the Ministry of science and technology(Grant No. 2016YFC0106802) and National Natural Science Foundation of China (Grant No. U1532107).
References
(1) Boras Z, Kondza G, Sisljagic V, Busic Z, Gmajnic R, Istvanic T. Prognostic factors of local recurrence and survival after curative rectal cancer surgery: a single institution experience. Coll Antropol. 2012;36(4):1355-61.
(2) Freedman L, Macaskill P, Smith A. Multivariate analysis of prognostic factors for operable rectal cancer[J]. The Lancet, 1984, 324(8405): 733-736.
(3) Akashi M, Nakahusa Y, Yakabe T, et al. Assessment of aggressiveness of rectal cancer using 3-T MRI: correlation between the apparent diffusion coefficient as a potential imaging biomarker and histologic prognostic factors. Acta Radiol. 2014;55(5):524-31.
Figures
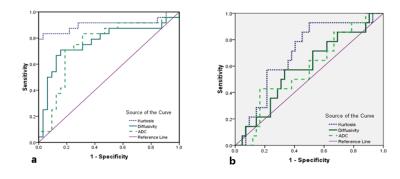
Figure 1: (a) Graph shows ROCs to assess performance of kurtosis, diffusivity and ADC in discriminating high- and low-grade rectal adenocarcinomas on the basis of number of PDCs
(b) Graph shows ROCs to assess performance of kurtosis, diffusivity and ADC in discriminating high- and low-grade rectal adenocarcinomas on the basis of WHO classification