1816
Evaluation of Motion-Compensated Spatially-Constrained IVIM (MC-SCIM) Model of Diffusion-weighted MRI for Assessment of Fibrosis in Crohn’s Disease using Surgical Histopathology Scores1Radiology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 2Pathology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 3Pediatrics, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
Distinguishing bowel regions with fibrosis and regions with active inflammation would be clinically useful in Crohn’s disease to determine best therapy. Commonly used ADC model of DW-MRI, which encapsulates multiple diffusion components into a single parameter, may not suffice to fully describe tissue microenvironments. IVIM model, which describes fast and slow diffusion components, is not commonly used in clinic because of challenges of reliably estimating its parameters due to noise and physiological motion. We recently introduced a motion-compensated spatially-constrained incoherent motion model (MC-SCIM) for reliable parameter estimation. Here we compared MC-SCIM parameters to scores of inflammation and fibrosis from histopathology.
Purpose
To evaluate ability of fast and slow diffusion components estimated using a motion-compensated spatially-constrained intra-voxel incoherent motion model (MC-SCIM) of diffusion-weighted MRI (DW-MRI) to assess fibrosis in Crohn’s disease (CD) using surgical histopathology scores.Introduction
CD is a chronic inflammatory bowel disease with frequent relapses and remits. Intestinal inflammation over time may progress to fibrosis and stricture formation1. A noninvasive quantitative measurement of bowel fibrosis would be clinically useful because the areas with stricture containing little or no fibrosis might respond to medical therapy while areas containing substantial fibrosis will benefit from surgery. MR Enterography (MRE) enables characterization of luminal narrowing in strictures but does not provide measures of fibrosis1. Previous work studied DW-MRI ADC parameter for fibrosis detection1. However, ADC model, which encapsulates the different signal decay components into a single parameter, may not be sufficient to fully characterize tissue microstructure. The bi-exponential intra-voxel incoherent motion (IVIM) model2 enables the characterization of the fast and slow diffusion components. However, reliable estimation of the IVIM parameters is very challenging due to the inherent noise and physiological motion. Our recently introduced technique improves IVIM parameter estimation reliability by 1) imposing a spatial homogeneity constraint on the model parameters and 2) by jointly compensating for motion and estimating parameters. In this work, we evaluate the ability of MC-SCIM3-5 technique for assessment of fibrosis and active inflammation in CD. We compare the parameters of slow diffusion and perfusion fraction with histopathology scores of surgically-resected regions.Methods
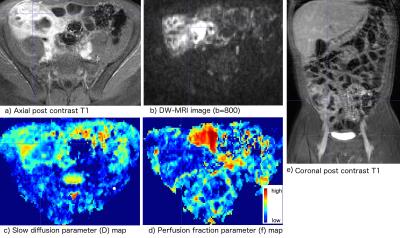
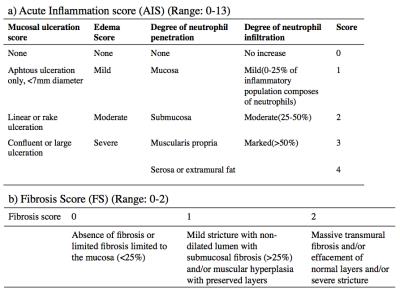
We collected 23 samples from surgically resected bowel tissues of 7 CD patients. We labeled the locations of collected samples on the gross digital picture of the resected tissue. The histopathology service prepared H&E stained tissue sections for evaluation. An experienced gastrointestinal pathologist graded the acute inflammation score (AIS) using the modified method of Borley et al.6 and the fibrosis score (FS) using the method of Chiorean et al.7 AIS and FS were graded on scales of 0-13 and 0–2, respectively. Before their surgery, patients were imaged using a standard MRE protocol including a DW-MRI sequence with 7 b-values on a 1.5T MRI scanner. An experienced radiologist located the tissue samples in the post-contrast T1w images of each patient. We aligned each b=0mm2/s image and post-contrast T1w image using rigid registration. We estimated the DW-MRI signal decay model parameters using our recently developed MC-SCIM technique, which uses a spatial homogeneity prior and estimates parameters for all voxels simultaneously, rather than solving for each voxel independently3,5. It also simultaneously solves image registration and model estimation problems by utilizing the interdependence of volumes along the diffusion-weighting dimension4. We estimated parameters of slow diffusion (D) associated with water molecule diffusion, fast diffusion (D*) associated with micro-capillary perfusion, and fraction of fast diffusion (f), associated with micro-capillary volume. We computed the average values of D and f parameters in regions labeled by the radiologist that are matched with the histopathology samples. The parameters were compared for regions with varying FS and AIS values.Results
Fig.1a) compares average and standard deviation of f parameter values in regions with varying FS values. The differences of f parameter values between regions with zero and non-zero FS were statistically significant (p<0.01) with f being lower in fibrotic regions. Some of those fibrotic regions also had varying amounts of AIS. Therefore, we also plotted in Fig.1b) and c) the average values of D and f parameters respectively in regions with four possible combinations of FS and AIS: 1) FS=0,AIS=0; 2) FS=0,AIS>0; 3) FS>0,AIS=0; 4) FS>0,AIS>0. The difference of D and f values were statistically significant (p<0.01) between category pairs labeled by * in Fig.1. Category 3 had only one sample. The f values were higher in regions with AIS>0,FS=0 than normal regions and lower in regions with FS>0 than normal regions (AIS=0,FS=0). Fig. 2 shows representative images from a patient, where indicated strictured region with FS>0,AIS>0 had lower f and D compared to normal-looking regions.Conclusions
Our results indicate that fast diffusion fraction (f) and slow diffusion (D) parameters estimated using the MC-SCIM technique are useful for identifying regions with fibrosis and active inflammation. The estimated parameters evaluated using the histopathology scores of fibrosis and inflammation had higher f in regions with inflammation and lower f in regions with fibrosis indicating reduction in microvasculature. Diffusion was restricted in regions with fibrosis and slightly more restricted in regions with inflammation only. Distinguishing regions with fibrosis and regions with active inflammation will be potentially useful to deciding upon the best possible therapy, including determining whether to use medical therapy, or surgery.Acknowledgements
This work is supported by the National Institute of Diabetes & Digestive & Kidney Diseases of the NIH under award R01DK100404. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
1. Barkmeier, D.T., Dillman, J.R., Al-Hawary, M. et al. MR enterography-histopathology comparison in resected pediatric small bowel Crohn disease strictures: can imaging predict fibrosis? Pediatr Radiol (2016) 46: 498.
2. Le Bihan, D., Breton, E., Lallemand, D., Aubin, M. L., Vignaud, J., & Laval-Jeantet, M. (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 168(2), 497-505.
3. Kurugol, S., Freiman, M., Afacan, O., Perez-Rossello, J. M., Callahan, M. J., & Warfield, S. K. (2016). Spatially-constrained probability distribution model of incoherent motion (SPIM) for abdominal diffusion-weighted MRI. Medical image analysis, 32, 173-183.
4. Kurugol, S., Freiman, M., Afacan, O., Domachevsky, L., Perez-Rossello, J. M., Callahan, M. J., & Warfield, S. K. (2015, October). Motion compensated abdominal diffusion weighted MRI by simultaneous image registration and model estimation (SIR-ME). In MICCAI (pp. 501-509). Springer.
5. Freiman, M., Perez-Rossello, J. M., Callahan, M. J., Voss, S. D., Ecklund, K., Mulkern, R. V., & Warfield, S. K. (2013). Reliable estimation of incoherent motion parametric maps from diffusion-weighted MRI using fusion bootstrap moves. Medical image analysis, 17(3), 325-33.
6. Chioran M.V., Sandrasegaran K., Saxena R., Maglinte D.D., Nakeeb A., Johnson C.S. (2007) Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol 102:2541–2550.
7. Dignass A., Van Assche G., Lindsay J.O., et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis 2010 Feb;4:28-62.
Figures