1813
Characterization of Spinal Cord DTI Metrics in Clinically Asymptomatic Pediatric Subjects with Incidental Congenital Lesions1Radiology, Thomas Jefferson University, Philadelphia, PA, United States, 2Bioengineering, Temple University, Philadelphia, PA, United States, 3Radiology, Temple University, Philadelphia, PA, United States, 4Occupational Therapy, Thomas Jefferson University, Philadelphia, PA, United States
Synopsis
Synopsis: Hydromyelia and syringomyelia are essentially cystic abnormalities of the spinal cord (SC). The prevalence of these abnormalities in the clinically normal pediatric population is uncommon to rare. Out of 26 healthy typically developing (TD) pediatric subjects scanned in this study, 4 subjects had incidental findings of hydromyelia (n=3) and syringomyelia (n=1) lesions within the thoracic SC. These subjects were healthy and clinically normal. DTI parameters were calculated by using ROIs drawn on the whole cord along the entire SC. DTI parameters were significantly different in the cord above the subject with syringomyelia lesion compared to the TD subjects. However, no significant difference in DTI parameters was found in the cord above the subjects with hydromyelia lesions. This study demonstrates that DTI has the potential to be used as an imaging biomarker to evaluate the SC above and below the congenital lesions in asymptomatic subjects and one should use caution while including them into a normative data population.
Purpose
Hydromyelia and
syringomyelia are essentially cystic abnormalities of the spinal cord (SC).
Hydromyelia is a dilatation of the central canal of the SC while
syringomyelia is defined as a fluid-filled cavity within the SC
parenchma1,2. DTI has been shown to
assess the microstructural changes in patients with syringomyelia3. However, the prevalence of these abnormalities in the clinically
normal pediatric population is uncommon to rare. Out of 26 healthy typically
developing (TD) pediatric subjects scanned in this study, 16% had unexpected
incidental findings on conventional MRI. These incidental findings were present
in the thoracic SC and represented hydromyelia lesion in 3 subjects and
syringomyelia lesion in 1 subject. Since these subjects were healthy and
clinically normal, the cord above and below the lesions, normal on MRI would be
expected to be normal on DTI as well. The purpose of this study was to
quantitatively analyze the cord in these subjects using DTI and comparing to the cord of the TD population. In this study, we performed both group analysis and
single-subject analysis to evaluate DTI characteristics of the cord.
Methods
Out of 26 TD recruited as part of large SC DTI study, 4 subjects had incidental findings of hydromyelia and syringomyelia. Written informed assent and consent was obtained under the protocol approved by IRB. Subjects underwent scans using a 3T MRI scanner. The protocol consisted of conventional T1- and T2-weighted structural scans and axial DTI scans based on inner field of view sequence4. The imaging parameters included: 3 averages of 20 diffusion directions, 6 b0 acquisitions, b=800s/mm2, voxel size=0.8x0.8x6mm3, axial slices=40, TR=7900ms, TE=110ms and TA=8:49min. After postprocessing5,6 FA, MD, AD and RD were calculated by using ROIs drawn on the whole cord along the entire SC. The subjects with hydromyelia had lesions from T3-T4 to T5-T6 levels (subject 1), Mid T6 to T10-T11 (subject 2) and T7-T8 to Lower L1 (subject 3) while subject with syringomyelia had lesion from Mid T3 to T4-T5 (subject 4). In all the subjects, the highest lesion was at Mid T3. Hence, for group analysis, mean values from C1 to T2-T3 were compared to the corresponding levels of the TD subjects. For single subject analysis, the cord above (subjects 1, 2, 3, 4) and below (subjects 1, 2, 4) the lesions was compared to the corresponding levels of the TD subjects respectively. Standard least squares fit model based on restricted maximum likelihood (REML) method was used. A p value ≤ 0.05 was statistically significant.Results
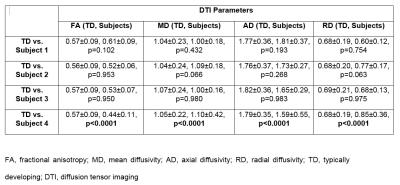
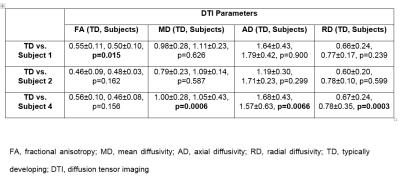
In the group analysis, MD and AD were significantly different in the cord compared to the TD subjects (Table 1). In single subject analysis, DTI parameters were significantly different in the cord above the subject with syringomyelia lesion compared to the TD subjects (Table 2). However, no significant difference in DTI parameters was found in the cord above the subjects with hydromyelia lesions (Table 2). In the cord below the hydromyelia lesion, no significant difference in DTI parameters was found except for FA in subject 1 (Table 3). In the cord below the syringomyelia lesion, MD, AD and RD were significantly different compared to TD subjects (Table 3).Discussion
In the clinically normal pediatric subjects, the apriori theory was that the cord above and below the congenital lesions should not be statistically different from the TD population. However, we found that the cord above the syringomyelia lesion demonstrated significant differences in DTI parameters which may correlate to microstructural changes including demyelination, and or axonal loss. Syringomyelia may have a different etiology, in comparison to hydromyelia, possibly representing a combination of congenital and acquired factors such as a subclinical prior demyelinating, traumatic or infectious or inflammatory process. The cord above the hydromyelia lesion showed no significant differences in DTI parameters suggesting that hydromyelia is likely a more benign process than syringomyelia. In the group analysis, MD and AD were significantly different in the cord above the lesion demonstrating that how a single subject (subject 4) can affect the mean of group results which may not correlate to a pathologic process. The limitation of this study is the small sample size. Future work with large number of subjects is needed to assess the DTI differences in the cord above and below these lesions.Conclusion
This study demonstrates that DTI has the potential to be used as an imaging biomarker to evaluate the SC above and below the congenital lesions in asymptomatic subjects and one should use caution while including them into a normative data population.Acknowledgements
This work was supported by National Institute of Neurological Disorders of the National Institutes of Health under award number R01NS079635.References
1. Johnston I, Teo C. Disorders of CSF hydrodynamics. Childs Nerv Syst 2000;16:776-99.
2. Ball MJ, Dayan AD. Pathogenesis of syringomyelia. Lancet 1972;14;2:799–801.
3. Yan H, Zhu Z, Liu Z, et al. Diffusion tensor imaging in cervical syringomyelia secondary to Chiari I malformation: preliminary results. Spine (Phila Pa 1976). 2015;40:E381-7.
4. Finsterbusch J. Improving the performance of diffusion-weighted inner field-of-view echo-planar imaging based on 2D-selective radiofrequency excitations by tilting the excitation plane. J Magn Reson Imaging 2012;35:984–92.
5. Middleton DM, Mohamed FB, Barakat N, et al. An investigation of motion correction algorithms for pediatric spinal cord DTI in healthy subjects and patients with spinal cord injury. Magn Reson Imaging 2014;32:433-39.
6. Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 2005;53:1088-95.
Figures