1802
Assessment of Inherent Variation in Diffusion Tensor Measurements with Multi-band EPI in Rhesus Monkeys1Yerkes Imaging Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States, 2Division of Neuropharmacology and Neurologic Diseases, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States
Synopsis
Multi-band EPI technique is becoming prevalent in diffusion MRI because it can reduce the scanning time substantially by simultaneously exciting multiple slices and then decoding signal by parallel MR imaging reconstruction algorithms. However, little is known about its influence on the inherent variations of diffusion measurements with or without multi-band parallel acquisitions. This study compared the variations of the in-vivo diffusion MRI measurement results by multi-band and conventional parallel acquisitions. The results showed that the inherent variations of diffusion MRI measurements can be reduced by multi-band parallel acquisition compared to conventional parallel MRI acquisition.
PURPOSE
To compare the inherent variations of the diffusion tensor imaging (DTI) measurements with multi-band technique and conventional parallel acquisition in in-vivo DTI of macaque monkeys at 3T.METHODS
Diffusion weighted images were acquired for adult rhesus monkeys (anesthetized with ~1.0% isoflurane) with a Siemens 3T TIM Trio scanner using 12-channel phased-array volume coil and an echo planar imaging (EPI) sequence with voxel size = 1.5 mm × 1.5 mm × 1.5 mm, FOV = 96 cm × 96 cm × 48 cm, and a single b-value of 1000 s/mm2 with 30 diffusion encoding directions, TE/TR = 76 ms/4 s for multi-band factor (MB) = 1 (conventional parallel acquisition), and TE/TR = 77 ms/3 s for MB = 3, GRAPPA acceleration factor = 2, and four averages for both acquisitions. To evaluate the inherent variations for the diffusion parameters derived from diffusion-weighted images, the diffusion-weighted signal of each diffusion direction was averaged across the whole brain, and then subtracted and divided by the signal averaged across the 30 diffusion directions, generating the relative signal intensity plotted in each diffusion direction. For both acquisitions with MB = 1 and MB = 3, FA maps were nonlinearly registered to a population-specific FA template, and then skeletonised with TBSS toolbox (FMRIB, Oxford). Mean diffusivity (MD) maps were also nonlinearly registered to the population-specific FA map and then skeletonized to produce the skeleton of the corresponding MD map. The histogram and the variations was calculated for FA and MD in the white matter skeletons for both DTI acquisitions (i.e., MB = 1 and MB = 3).RESULTS
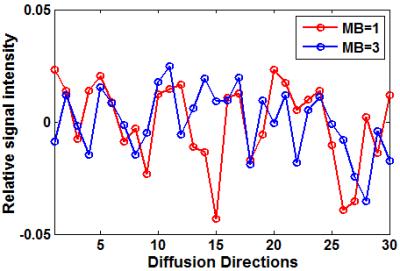
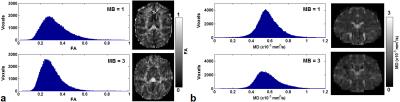
As seen in Figure 1, there were more signal fluctuations for the acquisition with MB = 1 than that with MB = 3, where the variations were 3.4×10-4 and 2.1×10-4 for MB = 1 and MB = 3 respectively. Figure 2 showed the distributions of FA and MD for both acquisitions. As compared to the results with MB = 1, with MB = 3 there were more voxels whose FA values appeared most often (Fig. 2a), while there were less voxels whose MD values appeared most often (Fig. 2b). Across the whole brain, the standard deviations of FA were 0.12 and 0.09 for the acquisitions with MB = 1 and MB = 3 respectively, and the standard deviations of MD were 1.37 ×10-3 mm2/s and 1.33 ×10-3 mm2/s for the acquisitions with MB = 1 and MB = 3 respectively.DISCUSSIONS
The results for this study showed that the inherent variations of DTI measurements were reduced with multi-band parallel acquisition 1. The improvement could be due to the reduction of excitations of multiple slices whose signal intensity could be varied for bulk and/or physiological motion during prolonged scanning 2. The reduced variation in FA/MD can benefit from reduced arbitrary signal frustrations. The reliability of DTI measures is essential for the preclinical and clinical applications of white matter disease model 3, especially in the cases of very limited nonhuman primate samples. Our results suggest that multi-band parallel acquisition could provide more robust measurement for quantitative DTI as compared with conventional parallel acquisition.Acknowledgements
Office of Research Infrastructure Programs / OD P51OD011132.References
1. Moeller S, Yacoub E, Olman CA, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63(5):1144–1153.
2. Walker L, Chang LC, Koay CG, et al. Effects of physiological noise in population analysis of diffusion tensor MRI data. Neuroimage. 2011;54(2):1168-77.
3. Cole JH, Farmer RE, Rees EM, et al. Test-Retest Reliability of Diffusion Tensor Imaging in Huntington's Disease. Plos Curr. 2014;6. pii: ecurrents.hd.f19ef63fff962f5cd9c0e88f4844f43b.