1788
Impact of Prior Distribution and Central Tendency Measure on Bayesian IVIM Model Fitting1Department of Radiation Physics, University of Gothenburg, Gothenburg, Sweden
Synopsis
Bayesian model fitting has been shown to yield robust estimates of the IVIM parameters. However, various methodological choices have differed between studies, which may have substantial effect on the results. This study investigates the effect that the prior distributions and central tendency measures may have, using both in vivo data and simulations. The results show that the prior distribution can play a significant role at commonly seen signal-to-noise levels. The choice of central tendency measure has less effect on the estimates. However, it may be chosen to emphasize either accuracy or precision.
Introduction and Purpose
Intravoxel incoherent motion (IVIM) MRI has gained attention due to its capability to simultaneously extract information about capillary perfusion and tissue water diffusion1,2. Following the theory of IVIM, signal decay due to application of diffusion weighting (quantified by b) is given by: $$S(b)=S_0((1-f)e^{-bD}+fe^{-bD^*})\qquad[1]$$ Where D is the diffusion coefficient, D* is the pseudo-diffusion coefficient, f is the perfusion fraction and S0 is the signal without diffusion weighting3. Due to the characteristics of the model and the data it is to describe, least squares estimation of IVIM model parameters is often hampered by low accuracy and precision. However, Bayesian model fitting has been proposed as a more robust alternative4,5. The objective of Bayesian model fitting is to estimate the posterior probability distribution of model parameters. According to Bayes’ theorem, an unnormalized posterior probability can be estimated given the likelihood function and the prior probability of the model parameters: $$\qquad P(\theta|Y)\propto P(Y|\theta)P(\theta) \qquad\qquad[2]$$ Where Y is data and θ is model parameters. By marginalizing the joint posterior distribution over all other model parameters, a distribution describing the posterior probability of a parameter is acquired. This distribution can be summarized in terms of central tendency to yield a scalar estimate. Both prior distribution and central tendency measure may influence the final parameter estimates and have differed between previous studies of Bayesian IVIM model fitting4-6. Therefore, the aim of this work was to study the impact of prior distribution and central tendency measure on Bayesian IVIM model fitting.
Materials and Methods
Model fitting
Three prior distributions and three central
tendency measures (mean6, median and mode4,5) were considered. The priors were:
1.
P(S0,f,D,D*) $$$\propto$$$ 1,
i.e. uniform distributions (used by e.g. Barbieri et al.4)
2. P(S0,f,D,D*) $$$\propto$$$ 1/(DD*),
i.e. reciprocal distributions for D and D* and uniform for f and S0 (used by
Neil et al.5)
3. P(S0,f,D,D*) $$$\propto$$$ 1/D*,
i.e. a hybrid between the previous two
The noise parameter was analytically marginalized using a reciprocal prior and Gaussian noise model7. Model parameter estimates were limited by setting the prior distributions to zero outside the bounds: D: [0 5]μm2/ms, f: [0 1], D*: [0 1000]μm2/ms and S0: [0 2Smax], where Smax is the maximum measured/simulated signal and by constraining D < D*. Marginal posterior distributions were estimated using a Markov-Chain Monte Carlo setup similar to Orton et al.6 with starting points given by segmented least squares fitting4 for in vivo data or true parameter values for simulations. The model fitting procedures given by the nine combinations of prior and central tendency measure were applied to the data described below to generate estimates of the IVIM model parameters.
In vivo measurements
Five nude mice, with subcutaneous human small
intestine neuroendocrine tumor in the neck region, were subjected to MR imaging in
a 7T system (Bruker). Diffusion-weighted SE-EPI
was performed with twelve different b-values
(1.4,5,10,20,35,50,75,100,201,401,602,802s/mm2, Δ=9ms, δ=4ms), TE=22ms
and voxel size=320µmx320µmx1000µm. The SNR without diffusion-weighting was
15-25 in the tumor tissue.
Simulations
To enable
an assessment of the accuracy of model parameter estimates 10,000 data series
with Rician noise (SNR 20 for b=0) were generated based on the IVIM model (eq.
1) using the same b-values as above. S0 was constant whereas the other model
parameters were randomly drawn from bounded uniform distributions given by D: [0.5 1.5]μm2/ms, f: [0 0.2] and D*: [10 100]μm2/ms. Ranges for D and f were based
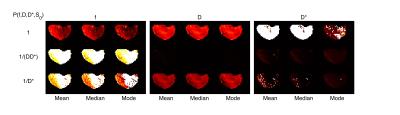
on the in vivo estimates using prior 1 (fig 1-3) and D* was taken from
literature4.
Results and Discussion
The IVIM effects of perfusion on the signal were in general small in all studied tumors, which made the estimation of IVIM parameters more challenging. Prior 2 and 3 tended to estimate high values of f and low values of D and D* compared with prior 1 (fig 1-2). The variability of parameter estimates was highly dependent on the magnitude of the parameter (fig 3).
The central tendency measures had a less pronounced effect on the parameter estimates although the mode tended to estimate slightly smaller values (fig 1-3). Simulation results agreed with in vivo results and showed that prior 1 had the best accuracy (fig 4) and had errors with smaller dependence on the magnitude of the model parameter values (fig 5).
Conclusion
Uniform prior distributions for all IVIM model parameters gave the best stability as well as highest precision and accuracy for estimates of f and D. Central tendency measure had less effect on the final result than the prior distribution, but can be chosen to emphasize either accuracy or precision.Acknowledgements
We are greatly thankful to Prof. Eva Forssell-Aronsson and Prof. Ola Nilsson for letting us use the GOT1 tumor model for the in vivo experiments.
This study was supported by research grants from the Swedish Cancer Society, the Swedish Research Council, the King Gustaf V Jubilee Clinic Cancer Research Foundation, the Sahlgrenska University Hospitals Research Foundations, the Assar Gabrielsson Foundation, the Wilhelm and Martina Lundgren science foundation, the Adlerbert student foundations and the Royal Society of Arts and Sciences in Gothenburg (KVVS).
References
1. Le Bihan D. Intravoxel Incoherent Motion Perfusion MR Imaging: A Wake-Up Call. Radiology. 2008;249:748-752.
2. Luciani A, Vignaud A, Cavet M, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology. 2008;249:891-899.
3. Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505.
4. Barbieri S, Donati OF, Froehlich JM, et al. Impact of the calculation algorithm on biexponential fitting of diffusion-weighted MRI in upper abdominal organs. Magnetic resonance in medicine. 2015;75:2175-2184.
5. Neil JJ, Bretthorst GL. On the use of Bayesian probability theory for analysis of exponential decay data: an example taken from intravoxel incoherent motion experiments. Magnetic resonance in medicine. 1993;29:642-647.
6. Orton MR, Collins DJ, Koh DM, et al. Improved intravoxel incoherent motion analysis of diffusion weighted imaging by data driven Bayesian modeling. Magnetic resonance in medicine. 2014;71:411-420.
7. Bretthorst GL, Hutton WC, Garbow JR, et al. Exponential Parameter Estimation (in NMR) Using Bayesian Probability Theory. Concepts in Magnetic Resonance Part A. 2005;27A:55-63.
Figures