1786
Automatic detection of volumes affected by subvolume movement1The Australian E-Health Research Centre, CSIRO, Brisbane, Australia, 2The University of Queensland, Brisbane, Australia
Synopsis
Diffusion-weighted MRI is prone to a number of artefacts, including movement between subvolumes in an interleaved acquisition. Affected volumes need to be identified and dealt with before further processing. We use a registration based approach to identify volumes affected by subvolume motion, and demonstrate that a single metric, calculated from all subjects acquired using the same acquisition protocol, is sufficient to reliably identify such volumes. Importantly, the detection threshold is determined from the data itself, and can be applied to multi-shell data.
Background
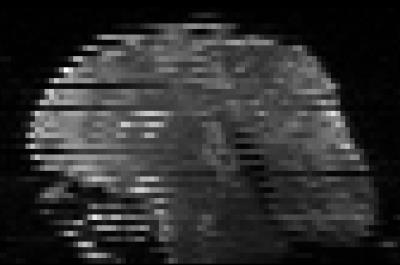
Diffusion-weighted images are typically acquired in an interleaved fashion. In difficult patient populations, e.g. neonates, this can lead to misalignment of the individually acquired subvolumes (Figure 1). We propose a registration-based approach to automatically detect volumes affected by within-volume motion.Methods
Preterm- and term-born infants underwent 3T MRI during natural sleep using an MR-compatible incubator with a neonatal head coil. MRI included 30-direction b=1000s/mm2 (DTI) and 64-direction b=2000s/mm2 (HARDI) diffusion MRI. Preterm-born infants were scanned around 30-32 weeks postmenstrual age (preterm-age [PTA] scan) and again around term equivalent age (TEA scan); term-born infants were scanned at TEA only. Five preterm-born and five term-born infants were randomly selected, each diffusion-weighted volume was visually inspected and labelled as containing within-volume motion or not. Individual volumes were divided into odd and even subvolumes and co-registered using rigid registration1. The root mean squared difference between the estimated transformations and the identity matrix was calculated for the registration of odd-to-even and even-to-odd subvolumes. The averaged root mean squared difference per volume was used as within-volume motion index. Within-volume motion indices were calculated separately for volumes manually labelled as containing within-volume motion, and compared to those calculated from volumes labelled as not containing within-volume motion. An acceptable threshold (t) of the within-volume motion index was calculated using the upper inner fence:
t = Q3 + 1.5 * IQR
where Q3 is the third quartile and IQR the inter-quartile range of within-volume motion indices across all volumes. Additionally, the performance of an iteratively calculated threshold was investigated. The iterative threshold was calculated as above, however using only values below the threshold. Thresholds were calculated (i) separately for each participant and diffusion acquisition; (ii) separately for each participant using the combined (“multi-shell”) diffusion acquisition; and (iii) for all participants combined using the combined diffusion acquisition. Performance was assessed by calculating sensitivity, specificity, positive predictive value, and F1 score.
Results
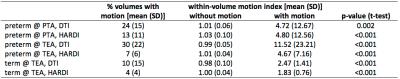
The percentage of volumes affected by motion (manual labelling), and within-volume motion indices observed in volumes with or without motion, is presented in Table 1. DTI data had more motion than HARDI data at all time points, likely due to either (i) DTI data being acquired after HARDI data, at which time the infants may have been more unsettled; and/or (ii) the higher signal to noise of the diffusion weighted images of the DTI data compared to the HARDI data, making it easier to visually identify volumes with motion for the DTI data. Within-volume motion indices of volumes without motion were consistent between DTI and HARDI, between time points, and between participant groups. Within-volume motion indices of volumes with motion were significantly higher, and had a wider spread.
The performance of the simple and iterative thresholds is reported in Tables 2. For single-shell data, the simple thresholds were markedly higher and more variable than iterative thresholds. Sensitivity was markedly increased for iterative thresholds, while specificity was slightly reduced, the positive predictive value was reduced, and F1 score was slightly increased. For multi-shell data, the difference between simple and iterative thresholds was only small, and very few differences are seen in sensitivity, specificity, positive predictive value and F1 score.
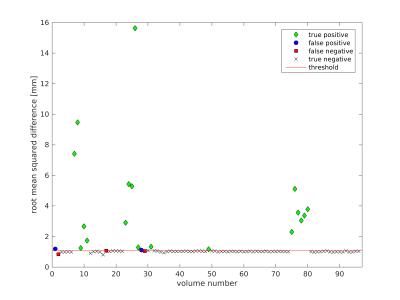
Because thresholds were similar across participants, it is reasonable to assume that a single threshold, calculated from all datasets together, could be used. This may be particularly useful for detecting motion on individual datasets that are heavily affected by motion: in those severe cases, the value of the obtained threshold (based on the individual dataset) might be artificially increased due to the increased presence of motion. The calculated simple threshold was 1.11, while the iterative threshold was 1.08 (example detection shown in Figure 2). The methods performed only slightly differently in terms of sensitivity and specificity, however the simple threshold performed better in terms of positive predictive value and F1 score.
Discussion
A single measure, the root mean squared difference between the transformation matrix and the identity matrix, was sufficient to successfully identify volumes affected by within-volume motion. Importantly, the threshold is determined from the data itself, with no need to set empirical thresholds as done previously2. The threshold can be calculated from the individual diffusion scan, or (more reliably in case of severe motion) from a group of participants acquired using the same protocol. It should be noted that the threshold depends on the acquisition parameters, in particular slice thickness. Volumes affected by subvolume motion can subsequently be removed from further analysis, included as subvolumes, or more advanced processing strategies can be performed, such as slice-to-volume registration3.Acknowledgements
The data used in this work were acquired as part of a project that received funding from the Cerebral Palsy Alliance Research Foundation, the Financial Markets Foundation for Children, the Queensland Government and the Royal Brisbane and Women’s Hospital.References
1. Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143-56
2. Liu, Z., Wang, Y., Gerig, G., Gouttard, S., Tao, R., Fletcher, T., & Styner, M. (2010). Quality control of diffusion weighted images. Proceedings of SPIE-the International Society for Optical Engineering, 7628. doi:10.1117/12.84474
3. Jiang, S., Xue, H., Counsell, S., Anjari, M., Allsop, J., Rutherford, M., Rueckert, D., & Hajnal, J. V. (2009). Diffusion tensor imaging (DTI) of the brain in moving subjects: Application to in-utero fetal and ex-utero studies. Magnetic Resonance in Medicine, 62(3), 645-55. doi:10.1002/mrm.22032
Figures