1784
Investigating the Effects of Concurrent Magnetic Field Monitoring on High Angular Resolution Diffusion Imaging: Application to Cortical Parcellation1Institute for Biomedical Engineering, ETH Zürich, Zürich, Switzerland, 2Laboratory for Social and Neural Systems Research, University of Zürich, Zürich, Switzerland, 3Department of Cognitive, Perceptual and Brain Sciences, University College London, London, United Kingdom, 4Centre for Medical Image Computing, University College London, London, United Kingdom, 5Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 6Psychology Department and Neuroimaging Center, San Diego State University, CA, United States
Synopsis
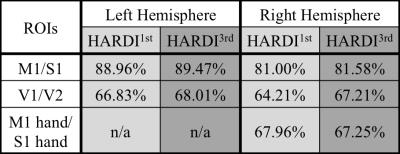
The concurrent field monitoring has been proposed to eliminate image artifacts in diffusion imaging introduced by the long lasting eddy currents from the diffusion-encoding gradients. In this work we investigated the effects of field monitoring system on HARDI and applied the improved HARDI data to cortical parcellation. We showed that the field monitoring improved the HARDI data quality especially in anterior/posterior poles of the brain and air-tissue interfaces. This regional improvement was also clear in the cortical classification results, where the field monitoring improved the accuracy of the V1/V2 by 3%, compared to ~0.5% in the motor strip.
PURPOSE
Undesirable time-varying components of magnetic field gradients lower image quality by introducing distortions, EPI ghosts and other image artifacts1. One way to eliminate these errors is to use magnetic field probes2 to measure the dynamic field variations3 and incorporate this information into the image reconstruction pipeline. Diffusion-weighted data acquisitions are notoriously susceptible to artifacts due to the long lasting eddy currents that are introduced by the diffusion-encoding gradients and affect the imaging gradients of the EPI readout train4. The aim of this abstract is to investigate the improvements provided by magnetic field monitoring in high angular resolution diffusion imaging (HARDI)5 acquisitions.METHODS
Data Acquisition: Using a 3T Philips Achieva scanner and 8ch receive-only head coil we collected HARDI data with 1 b0 image (b=0 s/mm2) and 61 DWIs (b=1000 s/mm2) on a healthy adult male volunteer (Table 1) under local ethics approval. Concurrently, the imaging gradient fields were measured by 16 magnetic field probes distributed around the head.
Image reconstruction: The HARDI data were reconstructed offline in two different ways: 1) The 0th and 1st order trajectory from the first slice of b0 image was estimated from the field probe data and used to reconstruct the b0 and HARDI data (HARDI1st). 2) Trajectories up to 3rd order were individually estimated for each slice of the b0 image and DWIs6 and used to correct for the higher-order field fluctuations and eddy currents from diffusion gradients (HARDI3rd).
Image processing: The two HARDI datasets were realigned for subject motion separately within ExploreDTI7. Subsequently, three different processing pipelines were used to investigate the improvement in the HARDI3rd data quality as compared to HARDI1st. First, we calculated the standard deviation (SD) across the diffusion-encoding direction8. Secondly, we calculated the voxel-wise residuals after fitting a tensor model to the data8. Finally, we carried out cortical parcellation9. Here, the HARDI datasets were sampled onto an existing FreeSurfer surface tessellation of the same volunteer, and a diffusion tensor feature vector was calculated at each vertex (FA, MD, RI10) for both datasets. We then extracted regions of interest, corresponding to the primary motor (M1), primary somatosensory (S1), primary (V1) and secondary (V2) visual areas using the Freesurfer Brodmann Atlas labels. In addition, two regions, corresponding to the M1 and S1 hand subdivisions, were used from the group average somatotopic data of 20 subjects11. In order to assess whether improvements in data quality provide higher classification accuracies, binary classification experiments of the above regions were performed, using random forest classification with leave-one-out cross validation.
RESULTS
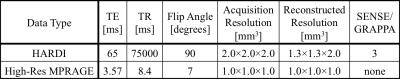
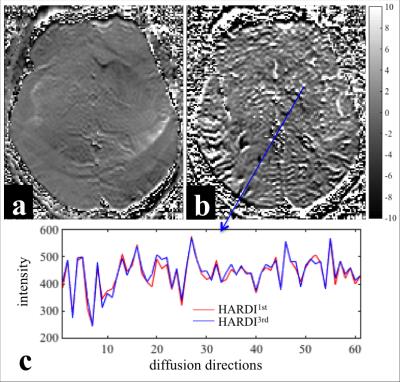
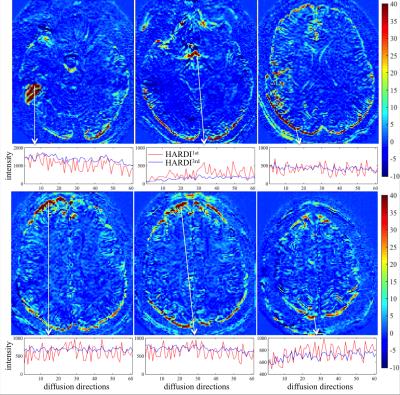
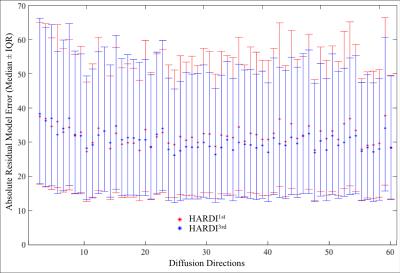
Figure 1 shows the difference map calculated by 100 × (HARDI1st – HARDI3rd) / HARDI3rd for a b=0 (a) and a b=1000 s/mm2 image (b) as well as the signal intensity evolution with the changing diffusion-encoding directions of the voxel near a subcortical gray/white matter boundary for both HARDI1st and HARDI3rd (c). Figure 2 shows the difference between HARDI1st and HARDI3rd SD maps for 6 different slices and the intensity variations from the voxels with prominent difference, e.g., edge of the brain and air-tissue interface. The median ± interquartile ranges of voxel-wise absolute residuals for voxels containing brain tissue in each diffusion direction are shown in Fig. 3. The percentages of correctly classified vertices for each binary classification experiment are shown in Table 2.DISCUSSION
Using higher order image reconstruction6, as afforded by the data collected with magnetic field probes2, has clear benefits for HARDI data quality. The benefits are regionally specific with the anterior/posterior poles of the brain and air-tissue interface areas benefitting the most. This regional effect is again clear in the cortical classification results, where the use of the field probe improves the accuracy of the V1/V2 by 3%, compared to ~0.5% in the motor strip. Note that image artifacts not related to the diffusion-encoding gradients are already corrected in all images through the use of 0th and 1st order trajectory. Please also note that V1 is not entirely in the high SD region (Fig. 2). Therefore, the 3% improvement in classification may be more significant. In voxels containing white matter, the changing diffusion directions modulate the signal extensively, therefore, calculating the SD may not be a good measure of data quality. The individually subtracted DWIs between HARDI1st and HARDI3rd highlight this aspect (Fig. 1a-c). For some diffusion directions the error can be up to 8%. Future work will investigate how the improved data quality with higher order reconstruction improves fiber tractography measures and also assess group-level impact of these improvements, presented here at the individual level.Acknowledgements
This work was supported by Swiss National Science Foundation (grant# 31003A_166118).References
1. Schmitt, F., Stehling, M. K., Turner, R., Schmitt, F., Stehling, M. K. & Turner, R. Echo-Planar Imaging: Theory, Technique and Application (Springer, 1998).
2. De Zanche, N., Barmet, C., Nordmeyer-Massner, J. A. & Pruessmann, K. P. NMR probes for measuring magnetic fields and field dynamics in MR systems. Magn Reson Med 60, 176-186 (2008).
3. Barmet, C., De Zanche, N. & Pruessmann, K. P. Spatiotemporal magnetic field monitoring for MR. Magn Reson Med 60, 187-197 (2008).
4. Wilm, B. J., Nagy, Z., Barmet, C., Vannesjo, S. J., et al. Diffusion MRI with concurrent magnetic field monitoring. Magn Reson Med 74, 925-933 (2015).
5. Jones, D. K., Horsfield, M. A. & Simmons, A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42, 515-525 (1999).
6. Wilm, B. J., Barmet, C. & Pruessmann, K. P. Fast higher-order MR image reconstruction using singular-vector separation. IEEE Trans Med Imaging 31, 1396-1403 (2012).
7. A. Leemans, A., Jeurissen, B., Sijbers, J., & Jones, D.K.. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proceedings of the 17th Annual Meeting of the International Society of Magnetic Resonance in Medicine, Hawaii, USA. p3537
8. Tournier, J. D., Mori, S. & Leemans, A. Diffusion tensor imaging and beyond. Magn Reson Med 65, 1532-1556 (2011).
9. Nagy, Z., Alexander, D. C., Thomas, D. L., Weiskopf, N. & Sereno, M. I. Using high angular resolution diffusion imaging data to discriminate cortical regions. PLoS One 8, e63842 (2013).
10. McNab, J. A., Polimeni, J. R., Wang, R., Augustinack, J. C., Fujimoto, K., Stevens, A., Janssens ,T., Farivar, R., Folkerth, R. D., Vanduffel, W. Surface based analysis of diffusion orientation for identifying architectonic domains in the in vivo human cortex. Neuroimage 6, 87–100 (2013).
11. Sood, M. and M.I. Sereno. Areas activated during naturalistic reading comprehension overlap topological visual, auditory, and somatotomotor maps. Human Brain Mapping 37, 2784-2810 (2016).
Figures