1780
Correction of nonuniform diffusion weighting in DWI using vendor-provided gradient characteristics1Radiology, University of Michigan, Ann Arbor, MI, United States, 2Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 3GE Global Research, Niskayuna, NY, United States, 4Siemens Healthcare, Malvern, PA, United States, 5Philips Research Laboratories, Hamburg, Germany, 6Radiology and Radiological Science, John Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
System-specific gradient nonlinearity (GNL) causes spatially nonuniform weighting in diffusion weighted imaging (DWI). This leads to systematic bias and variability in derived apparent diffusion coefficient (ADC) maps, diminishing their quantitative utility for multi-site, multi-platform clinical trials. An ADC error correction methodology for three-direction DWI acquisition was developed previously using an empiric system GNL approximation. Here we demonstrate implementation of correction for three clinical scanners using the system-specific gradient-channel fields derived from vendor-provided spherical harmonic tables. Implemented correction substantially improves precision and removes ADC bias for ice-water phantoms. Comparable accuracy and performance is achieved across all gradient platforms.
Introduction
Spatial nonuniformity of diffusion weighting (DW) induced by system-specific gradient nonlinearity (GNL)1 confounds apparent diffusion coefficient (ADC) maps for off-center anatomy. This platform-dependent bias causes significant errors and variability in ADC measurements in multi-site, multi-platform clinical trials that utilize quantitative DWI2,3. Previously, a framework was developed4 to correct for the bulk of systematic ADC bias error for a mono-exponential medium of arbitrary anisotropy using three orthogonal DWI measurements. In the present work, the proposed DW bias correction was implemented for three clinical scanners using gradient channel design information provided by vendors.Methods
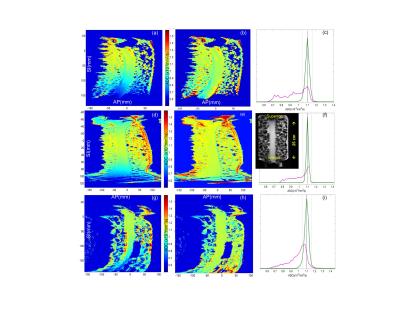
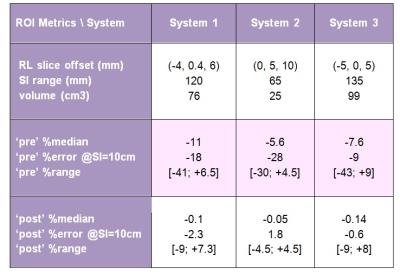
Sagittal DWI scans of a long-tube ice-water phantom2 (Figure1f, insert) were performed on Siemens Espree, GE Signa, and Philips Ingenia 1.5T MRI scanners, using three b-values (b = 0, 750, 1500), with three DWI gradient directions, uk, along primary magnet axes. Scanner-specific nonlinearity tensors, L(r),1 were calculated numerically on a 4-5 mm 3D grid within the magnet bores (450-600 mm diameter) by each site using shared Matlab libraries and gradient design (spherical harmonics) coefficients1 provided by their vendors. Three-dimensional bias corrector maps for each gradient direction were generated by the central analysis site as, Ck(r) = Tr(Luk[Luk]T)4. For experimental ice-water DWI data, the directional-average correctors were 3D-spline interpolated according to the DICOM header information for each imaged volume and resolution. The 3D corrector maps were then applied on a pixel-by-pixel basis to yield corrected trace-DWI intensities and b-maps to derive unbiased ADC4. ADC maps were obtained from the linear log-signal DWI fit for (b = 0, b > 0) pairs. The ADC bias, %(ADC- ADC0)/ADC0, was estimated as percent-deviation from the known diffusion value of ice-water, ADC0 = 1.1 (x10-3mm2/s)5. ADC regions-of-interest (ROIs) were defined on three slices of the phantom tube near zero right-left (RL) offset (Table 1) and superior-inferior offset SI > 30mm. The correction performance was quantified by reduction of percent-bias for ROI histogram metrics (median and range).Results
The observed ADC bias was independent of b-value, ranged from -43% to +9% and induced systematic variations across all systems: e.g., about 9% at SI = 10cm (Table 1). The steep DW nonuniformity along SI phantom tube, Fig.1 (a,d,g), resulted in systematic (skewed) broadening of the corresponding ADC histograms, Fig.1 (c,f,i, magenta). Application of the system-specific corrector maps substantially improved ADC uniformity, Fig.1 (b,e,h), and reduced the histogram widths (down to ±5% range for 90th percentile, Fig.1 (c,f,i, green)). The median bias error was effectively eliminated (<0.1%) and systematic cross-platform variability along SI was reduced (e.g., about 2% at SI=10cm after correction, Table 1) to below measurement noise <3%. Both original and residual histogram widths depended on phantom volume and SI extent of the selected ROI (Table 1).Discussion
Consistent with previous observations,2,3 uncorrected DW nonuniformity led to substantial errors both in absolute ADC values at offset locations and technical cross-system variability. The GNL-bias correction was clearly necessary to improve precision, uniformity and reproducibility of ADC maps at off-center locations. Adequate correction performance was achieved across all studied systems. The described results confirmed theoretical prediction of static character for GNL-induced nonuniformity bias in b-value1,4 determined primarily by the gradient system design. As shown, once built for a specific gradient system, the corrector maps can be applied to the DWI scans of arbitrary object and geometry. Analogous to commonly-used correction of geometric distortion, the prospective correction of GNL-induced DW bias can be implemented on-scanner using gradient system design and DWI scan geometry information.Conclusion
The vendor-provided gradient channel descriptions adequately accounted for observed systematic b-value nonuniformity bias across the three studied clinical scanner platforms. For a temperature-controlled phantom, implementation of GNL bias correction substantially enhanced the accuracy of ADC map values and eliminated systematic cross-platform variability. These results demonstrate feasibility of prospective (on-scanner) correction for the system-specific GNL-induced bias in diffusion weighting.Acknowledgements
This research was supported by National Institutes of Health Grants: R01CA190299, U01-CA166104, U01CA151235, U01CA140204, 5P30CA006973.References
1Bammer R, Markl M, Barnett A, et.al. Analysis and generalized correction of the effect of spatial gradient field distortions in diffusion-weighted imaging. Magn Reson Med. 2003;50(3):560-9.
2Malyarenko DI, Newitt D, Wilmes LJ, et.al. Demonstration of nonlinearity bias in the measurement of the apparent diffusion coefficient in multicenter trials. Magn Reson Med. 2016;75(3):1312-23.
3Newitt DC, Tan ET, Wilmes LJ, et al. Gradient Nonlinearity Correction to Improve Apparent Diffusion Coefficient Accuracy and Standardization in the American College of Radiology Imaging Network 6698 Breast Cancer Trial. J Magn Reson Imaging. 2015 Oct; 42(4): 908–919
4Malyarenko DI, Ross BD, Chenevert TL. Analysis and correction of gradient nonlinearity bias in apparent diffusion coefficient measurements. Magn Reson Med. 2014;71(3):1312-23.;
5Holtz M, Heil SR, Sacco A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate H-1 NMR PFG measurements. Phys Chem Chem Phys 2000;2(20):4740-42.
Figures