1779
Prediction of outcome in bilateral common carotid artery occlusion (BCCAO) rats by intravoxel incoherent motion (IVIM) analysis at 11.7 Tesla1Department of Neurosurgery, Iwate Medical University, Morioka, Japan, 2WPI Immunology Frontier Research Center, Osaka University, Suita, Japan, 3Center for Information and Neural Networks (CiNet), NICT and Osaka University, Suita, Japan
Synopsis
Rats with the bilateral carotid artery occlusion (BCCAO) was often used for assessment of the brain damage caused by chronic cerebral hypoperfusion as a longitudinal ischemic animal model; however, the mortality is high and it has remained unclear what kinds of initial cerebral hemodynamic changes occurred in the brain in the hyperacute phase after BCCAO and whether the changes related with the mortality in rats or not. Intravoxel incoherent motion (IVIM), which is the basic concept of diffusion-weighted imaging (DWI), can non-invasively demonstrate various hemodynamic situations at one time DWI scan with multiple b values. Here, we investigated whether the outcome of BCCAO rats associated with cerebral hemodynamic changes assessed using IVIM- DWI.
INTRODUCTION
For assessment of the longitudinal brain damage caused by chronic cerebral hypoperfusion, rats with the bilateral carotid artery occlusion (BCCAO) have been used1-4. BCCAO model has an advantage that cerebral hemodynamic changes and post-ischemic neural degenerations can be observed without serious surgical injuries differing from other ischemic models like the middle cerebral artery occlusion model. On the other hand, the BCCAO model has shown a high mortality rates, which was improved by a staged ligation3,4 in which the second ligation of a carotid artery was performed 2-7 days after the first one. However, it has remained unclear what kinds of initial cerebral hemodynamic changes occur in the hyperacute phase after BCCAO and whether these changes relate to high mortality rates or not. Intravoxel incoherent motion (IVIM), which is the basic concept of diffusion-weighted imaging (DWI), can non-invasively assess various hemodynamic situations and the IVIM-DWI can be performed at one-time scan with multiple b values5,6. Here, we investigated whether the outcome of BCCAO rats associated with cerebral hemodynamic changes assessed using IVIM- DWI.METHODS
Animals: We performed a surgical treatment to 10 female Wistar rats (8 week-old, initial body weight, 156.4±7.1 g) as follows: first, a right common carotid artery (CCA) was occluded by a ligation with a 4-0 surgical thread; second, a left CCA was occluded 6 days after the previous unilateral occlusion. Rats that survived until the 3 week after the occlusion were assigned to a long survival (LS) group, while the other rats were grouped in a non-LS group.
MRI: IVIM-DWI (multi-shot spin echo EPI sequence; matrix, 64×128; field of view, 12.8×25.6 mm2; in-plane resolution: 0.2×0.2 [mm2]; slice thickness: 0.8 [mm]; 12 b values: 0, 10, 20, 40, 80, 160, 320, 640, 800, 1000, 2000, 3000 [s/mm2]; motion probing gradient, 6 directions) was performed on a preclinical vertical 11.7 Tesla MRI scanner (AVANCE II 500WB, Bruker) before a surgical treatment and 1 hour after the second ligation.
Analysis: For qualitative assessments, IVIM parameter maps were obtained from each rat by estimating the values pixel by pixel using an exhaustive search method with the DWI signal database without curve fitting procedures7. IVIM parameters were then quantitatively estimated from the averaged signal in a region of interest (ROI) automatically located on the left and right cortex in each rat by our developed software. The ratio of each parameter after BCCAO to that before BCCAO (a/b ratio) was calculated.
Statistics: The significant difference in each IVIM parameter between LS and non-LS group was examined using Mann-Whitney U test. Receiver operating characteristic (ROC) curve analysis was performed to define an optimized each IVIM parameter for distinguishing rats in the LS group from those in the non-LS group. All statistics were performed with the significant level p<0.05.
RESULTS
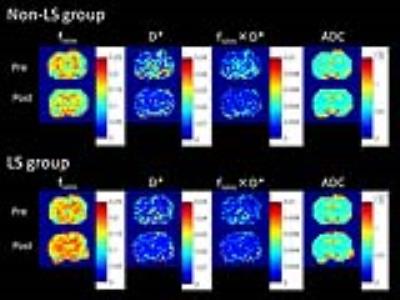
MRI scans before and after BCCAO were successfully performed in all rats. Four of ten rats (40%) were assigned to a long survival (LS) group, while the other six rats (60%) were grouped in a non-LS group. Typical IVIM parameter maps in each group are shown in Figure 1. In quantitative assessments, the a/b ratio of fivim (median: LS, 1.07; non-LS, 0.94) was significantly higher (p=0.002) in the LS group than in the non-LS group. The a/b ratio of fivim×D* (LS, 1.32; non-LS, 0.87) was lower (p=0.04) in the non-LS group than in the LS group. On the other hand, there were no significant differences in the a/b ratios of D* and ADC. ROC analysis (area under curve = 0.911; p<0.0001) showed that the a/b ratio of fivim was a good parameter to predict the mortality at the 3 week when the cut-off value was 0.99 (sensitivity, 100%; specificity, 75%; positive predictive value, 73%; negative predictive value, 100%).
DISCUSSION
We found that fivim elevated immediately after BCCAO in LS rats, clearly separating non-LS rats with the cut-off value. The fivim elevation indicates that CBV in LS rats might be elevated at the hyperacute phase. No study has assessed cerebral hemodynamic changes in BCCAO rats using IVIM-DWI. Although we need further studies to quantitatively validate the changes using 15O positron emission tomography, the hyperacute fivim elevation indicates that the collateral flow to relieve the severe ischemic damage caused by BCCAO might exist in the LS rats more than in the non-LS rats.CONCLUSION
IVIM parameter in the hyperacute phase after BCCAO in rats can be a good indicator to predict the outcome until the 3 week.Acknowledgements
This study was supported in part by Grant Program for Biomedical Engineering Research from Nakatani Foundation, Grant-in-Aid for Scientific Research (C) (No.15K09935, 2015-2018) and Grant-in-Aid for Strategic Medical Science Research (S1491001, 2014-2018) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
1. Ouchi Y, Tsukada H, Kakiuchi T, Nishiyama S, Futatsubashi M. Changes in cerebral blood flow and postsynaptic muscarinic cholinergic activity in rats with bilateral carotid artery ligation. J Nucl Med. 1998;39(1):198-202.
2. Ihara M, Tomimoto H, Kinoshita M, et al. Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of white matter. J Cereb Blood Flow Metab. 2001;21(7):828-834.
3. Fateev IV, Bykov VN, Chepur SV, et al. A model of cerebral circulation disorders created by staged ligation of the common carotid arteries. Bull Exp Biol Med. 2012;152(3):378-381.
4. Jing Z, Shi C, Zhu L, et al. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab. 2015;35(8):1249-1259.
5. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168(2):497-505.
6. Le Bihan D, Turner R. The capillary network: a link between IVIM and classical perfusion. Magn Reson Med. 1992;27(1):171-178.
7. Fujiwara S, Uhrig L, Amadon A, Jarraya B, Le Bihan D. Quantification of iron in the non-human primate brain with diffusion-weighted magnetic resonance imaging. NeuroImage 2014;102P2:789-797.