Fenglei Zhou1,2, Matthew Grech-Sollars3, Adam Waldman3,4, Geoffrey J. M. Parker1,5, and Penny L. Hubbard Cristinacce6
1Division of Informatics, Imaging & Data Sciences, School of Health Sciences, The University of Manchester, Manchester, United Kingdom, 2The School of Materials, The University of Manchester, Manchester, United Kingdom, 3Division of Brain Sciences, Imperial College London, London, United Kingdom, 4Centre for Clinical Brain Sciences, The University of Edinburgh, Edinburgh, United Kingdom, 5Bioxydyn Limited, Manchester, United Kingdom, 6Division of Neuroscience & Experimental Psychology, The University of Manchester, United Kingdom
Synopsis
This work
investigates the stability and reproducibility of brain-mimicking microfiber phantoms.
These microfibers were produced by co-electrospinning (co-ES) and characterized
by scanning electron microscopy (SEM). Grey matter (GM) and white matter (WM)
phantoms were constructed from random and aligned microfibers, respectively. MR
data were acquired from these phantoms over a period of 17 months. SEM images reveal that
there were some changes in the pore size and porosity of co-ES fibers over a
period of 30 months. MR measurements showed variations within the limits
expected for intra-scanner variability, thereby confirming the phantom
stability over 17 months.
Purpose
The design of
physical phantoms requires appropriate chemical stability and reproducibility. We
have created brain-mimicking hollow microfibers by co-ES.1 The
resultant microfibers have previously been used as a building block to
construct WM 2, GM 3, and cardiac phantoms 4 for
validating diffusion magnetic resonance imaging (dMRI) methods. Our previous
studies have shown the short-term (1–4 weeks) stability and reproducibility of
MR phantoms.1,4 This study presents the SEM-derived microstructural
changes over 30 months and MR measurements of co-ES phantoms acquired over 17
months.
Methods
Aligned and random polycaprolactone (PCL) hollow microfibers with small
and large inner diameters were produced in the optimized co-ES processes using 0.8
and 2.0 mL/h core flow rates.
1 The cross sections of co-ES fibers
were observed by SEM after freeze fracture. SEM images were acquired at each
time point – raw, 1, 3, 6, 12, 24 and 30 months (only WM phantoms). Pore size
and porosity (pore area/total area) were calculated using ImageJ by converting
an SEM image to binary image and thresholding.
5
A number of aligned strips and random mesh layers were packed into glass
tubes filled with cyclohexane, and these were used as small and large WM (1 and
2) and GM (1 and 2) phantoms, respectively. Four sets of PGSE MR diffusion
tensor imaging data (b=0,1000 mm
2/s; 30 directions) for the four
phantoms; two representing GM’s globally isotropic structure and two
representing WM’s anisotropic structure, were acquired on a 1.5 T Siemens
Avanto clinical MRI system over a period of 17 months.
Results
Fig. 1a and b reveal
that the cross sections of aligned fibers remain porous across 30 months. The
two dominant characteristic structural features are pores and merging fibers. Fig.1c
and d show porous random cross section of the GM fibers when dry at 0 month
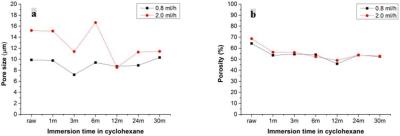
(raw) and at 25 months. Fig. 2 shows area-weighted pore size and porosity of two
fiber types at 7 time points. The pore sizes of two fiber types (Fig. 2a) show
there are variations between 3 and 22%, 10 and 34%, respectively, possibly due
to variability in the semi-automated sampling and measurement process. Fig. 2b demonstrates
the corresponding porosity, which varied approximately 20% or less over 30
months.
WM
phantoms 1 and 2 had the pore sizes of 10.1 μm and 13.7 μm (area-weighted),
respectively. GM phantoms 1 and 2 had the pore sizes of 8.0 μm and 2.7 μm (area-weighted),
respectively. MR results (Fig. 3) for mean diffusivity (MD) and fractional
anisotropy (FA) showed a coefficient of variation (CV) between 1.1 and 4.3%
(mean 2.6%) for MD (Fig. 3a) in all phantoms and for FA (Fig. 3b) in the WM phantoms,
comparable to the variability expected in repeat scans within the same session as
previously reported.
6 The CV for FA in GM was higher (8% in one
phantom and 15.2% in the other), as expected in media with a naturally low
value of FA.
6Discussion
Biomimetic
phantoms developed by co-ES were stable over 30 months on SEM. The
cross-section pores can be classified into intrafiber pores, interfiber pores and
void pores
5, each of which has distinct features. Among these
pores, only intrafiber pores are controllable by co-ES parameters. Pore
distortion resulting from the freeze fracture method produced inevitable errors
with the calculated diameters and porosity. There is a certain degree of
variability with immersion time possibly due to the variability in the analysis
method and sampling. Additionally, large number of outliers and extremes (not
shown) were observed indicating that void pores were present in most of aligned
fiber samples, which contributes to the variability between immersion time
points. The ≤20% variation in porosity indicates that co-electrospun fibers
were reasonably stable in cyclohexane. There is a significant difference in
pore sizes between two WM phantoms, which is responsible for the differences in
MD and FA. Imaging measurements for MD and FA showed variations within the
limits expected for intra-scanner variability, thereby confirming the phantom
stability over the 17 months. The SEM shows some evidence of changes in pore
size and porosity over 30 months, but these were not reflected in the MD and FA
measurements.
Conclusion
We envisage that
co-ES brain-mimicking phantoms will be used for the validation of
novel and established diffusion MRI methods, as well as for routine quality
assurance purposes and for establishing scanner performance in multicentre
trials. To our knowledge these are the first synthetic, controllable phantoms
mimicking the layered structure of grey matter for dMRI.
Acknowledgements
FLZ and MGS contributed
equally to this work. This research was supported by "CONNECT”, the FET
Programme (FET-Open grant number: 238292), The Brain Tumour Charity, the Brain
Tumour Research Campaign, and the CRUK and ESPRC Cancer Imaging Centre in
Cambridge and Manchester (C8742/A18097).References
1. Zhou FL, Hubbard PL, Eichhorn SJ, Parker GJM. Coaxially
electrospun axon-mimicking fibers for diffusion magnetic resonance imaging. ACS Appl Mater. Interfaces. 4(2012),
6311-6316.
2. Hubbard
PL, Zhou FL, Eichhorn SJ, Parker GJM. Biomimetic phantom for the validation of
diffusion magnetic resonance imaging. Magn Reson Med.73 (2015), 299-305.
3. Allen AQ, Hubbard
Cristinance PL, Zhou FL, Yin Z, Parker GJM, Magin RL. Diffusion tensor MRI
phantom exhibits anomalous diffusion. IEEE EMBS Proceedings, 2014, 746-749.
4. Teh I, Zhou FL, Hubbard
Cristinacce PL, Parker GJM, Schneider JE. Biomimetic phantom for cardiac diffusion MRI. J Magn Reson Im.43 (2015), 594-600.
5. Zhou FL, Eichhorn SJ, Parker GJM, Hubbard Cristinacce PL, Production and cross-sectional
characterization of aligned co-electrospun hollow microfibrous bulk assemblies.
Mater Charact. 109 (2015), 25-35.
6. Grech-Sollars M,
Hales PW, Miyazaki K, et al. Multi-centre reproducibility of diffusion MRI
parameters for clinical sequences in the brain. NMR Biomed. 28(2015), 468-485.