1763
Accelerated Diffusion-weighted 129Xe MRI Morphometry of Emphysema in COPD and Alpha-1 Antitrypsin Deficiency Patients1Robarts Research Institute, London, ON, Canada, 2Division of Respirology, University of Western Ontario, London, ON, Canada
Synopsis
In this proof-of-concept evaluation, we evaluated 129Xe MRI ADC/morphometry estimates using two different acceleration factors (AF) in a small group of never-smokers, COPD ex-smokers with emphysema and Alpha-1 Antitrypsin Deficiency patients. Such estimates were obtained for three different cases: fully sampled k-space; 50% under-sampling in the phase-encoding direction, AF=2; and 66% undersampling, AF=3. The results of this study showed that the difference in ADC/morphometry estimates from fully sampled and under-sampled k-space were similar to that observed with accelerated 3He multi-b diffusion-weighted MRI in healthy subjects. These differences increase however, with increasing emphysema severity, which requires further investigation.
Purpose
Hyperpolarized 129Xe pulmonary MRI is poised for clinical-translation due in part to the clinical-relevance of 129Xe MRI biomarkers of lung-disease, a stable supply of 129Xe gas and the commercial availability of polarizers that generate large volumes of highly polarized gas.1 The physical properties of 129Xe gas, however, dictate rapid MRI acquisition strategies. This is especially true in the case of multi-b diffusion-weighted MRI because currently, a full data-set cannot be acquired during the relatively short 10-18s breath-holds that are clinically-feasible.2 Whole-lung 3D multi-b diffusion-weighted data in a single breath-hold was shown feasible for 3He lung MRI using traditional k-space sampling,3 parallel-imaging4 and compressed-sensing (CS).5 We hypothesized that accelerated 129Xe lung MRI will also permit acquisition of whole lung 3D multi-b diffusion-weighted data-set in a single 16s breath-hold and similar to the spatial resolution of 3He MRI results. Therefore, in this proof-of-concept evaluation, our objective was to evaluate ADC and morphometry estimates in a small group of never-smokers, COPD ex-smokers with emphysema and Alpha-1 Antitrypsin Deficiency (AATD) patients. The estimates were obtained for three different cases: 1) fully sampled k-space, 2) 50% under-sampled k-space in the phase-encoding direction,6 (acceleration factor (AF)=2, Figure 1a), and 3) 66% under-sampled k-space (AF=3,5 Figure 1b).Methods
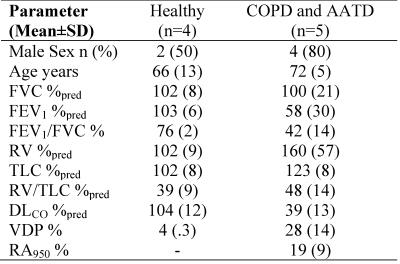
As shown in Table 1, nine participants (four never-smokers, four COPD and one AATD patient) provided written-informed-consent to an ethics-board-approved protocol and underwent spirometry, plethysmography, CT and 129Xe MRI. Imaging was performed at 3.0T (MR750, GEHC, Milwaukee, WI) using whole-body gradients (5G/cm maximum) and a custom-built, rigid quadrature unshielded asymmetrical RF coil.2 In a single breath-hold (AATD case), four interleaved acquisitions (3D FGRE, VFA, TE/TR=9.0ms/10.0ms, matrix-size=64x64, number-of-slices=7; slice-thickness=30mm, and FOV=40x40cm2) with and without diffusion-sensitization were acquired for a given line of k-space. For all other subjects, two interleaved acquisitions (2D FGRE, TE/TR=9.8ms/11.0ms, matrix-size=128x128, number-of-slices=7; slice-thickness=30mm, and FOV=40x40cm2) with and without diffusion-sensitization were acquired for a given line of k-space to ensure that RF depolarization (5o constant flip-angle was used) and T1-relaxation effects were minimal.2 The diffusion-sensitization gradient pulse ramp up/down-time=500μs, constant-time=2ms, diffusion-time (Δ)=5ms, providing four b-values 0, 12.0, 20.0, and 30.0s/cm2. Hyperpolarized 129Xe (86% enriched, polarization~12-20%) was provided by a xenon polarizer system1 (XeniSpin™, Polarean, Durham, NC). 1L of a 50/50 hyperpolarized 129Xe/4He gas mixture was inhaled from functional residual capacity. For COPD and never-smokers participants, a single 1L mixed dose was inhaled.2 Two k-space masks mimicking AF=2 and AF=3 were applied to fully sampled k-space (each b-value) in order to obtain under-sampled k-spaces with two different acceleration factors. The Projection-onto-Convex-Sets6 and CS7 were used to reconstruct diffusion-weighted images from under-sampled k-spaces with AF=2 and AF=3, respectively. 129Xe lung morphometry maps (diffusivity [DDC], heterogeneity-index [α] and mean-linear-intercept [Lm]) were estimated using the stretched exponential method5,8 ($$$S(b)/S_{0}=\int_{0}^{D_{0}}P(D) exp(-\bf b\cdot\it D)dD$$$, which provides the distribution of length scales (LD=[2ΔD]1/2)) which was extended to provide the clincally-relevant estimates such as a mean linear intercept9 and adapted for 129Xe. The morphometry maps for AF=2/AF=3 and full sampling along with two b-value (0 and 12s/cm2) ADC, were computed on a voxel-by-voxel basis.9Results
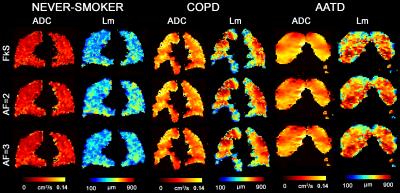
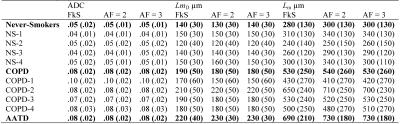
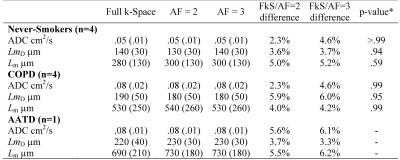
Figure 2 shows representative centre slices using all three sampling methods for ADC and Lm maps for a single never-smoker and COPD/AATD patients. Table 2 shows mean estimates ADC, Lm, and mean airway length scale10 (LmD) for all subjects. Table 3 shows overall mean estimates of ADC, LmD and Lm and corresponding differences. Mean ADC, and Lm estimates for the never-smokers (0.05cm2s-1/280µm) were significantly smaller than the corresponding mean estimates for COPD and AATD patients (0.08cm2s-1/600µm; all p<.001) for all three sampling methods. For never-smokers, mean differences of 1.8%/5.0% and 2.2%/5.2% were observed between fully sampled and under-sampled (AF=2/AF=3) k-space ADC and Lm values, respectively. For the COPD subgroup, a mean difference of 2.3%/4.6% and 4.0%/4.2% was observed between fully sampled and under-sampled (AF=2/AF=3) k-space ADC and Lm values, respectively. For the AATD subject, a mean difference of 5.6%/6.1% and 5.5%/6.2% observed between fully sampled and under-sampled (AF=2/AF=3) k-space ADC and Lm values, respectively.Discussion
The results of this proof-of-concept study show that the difference in ADC and LmD estimates obtained from the fully sampled and under-sampled k-space were similar to observed with accelerated 3He multi-b diffusion-weighted MRI in healthy subjects.5 These differences as well as Lm differences increase however, with increasing emphysema severity. Therefore, while promising, accelerated multi-b diffusion-weighted lung MRI 129Xe morphometry in patients with severe emphysema requires further investigation.Conclusion
In this proof-of-concept study, preliminary results show the feasibility of accelerated diffusion-weighted 129Xe MRI morphometric measurements in COPD and AATD, but further studies are necessary in patients with severe emphysema.Acknowledgements
A. Ouriadov gratefully acknowledges fellowship support from the Alpha-one Foundation (USA). We also thank Trevor Szekeres, RTMR and Dave Reese, RTMR for MRI of research volunteers.References
1 Cleveland, Z. I., Möller, H. E., Hedlund, L. W. & Driehuys, B. Continuously Infusing Hyperpolarized (129)Xe into Flowing Aqueous Solutions Using Hydrophobic Gas Exchange Membranes. The journal of physical chemistry. B 113, 12489-12499, doi:10.1021/jp9049582 (2009).
2 Ouriadov, A. et al. Lung morphometry using hyperpolarized (129) Xe apparent diffusion coefficient anisotropy in chronic obstructive pulmonary disease. Magn Reson Med 70, 1699-1706, doi:10.1002/mrm.24595 (2013).
3 Paulin, G. A. et al. Noninvasive quantification of alveolar morphometry in elderly never- and ex-smokers. Physiol Rep 3, doi:10.14814/phy2.12583 (2015).
4 Chang, Y. V., Quirk, J. D. & Yablonskiy, D. A. In vivo lung morphometry with accelerated hyperpolarized (3) He diffusion MRI: a preliminary study. Magn Reson Med 73, 1609-1614, doi:10.1002/mrm.25284 (2015).
5 Chan, H. F., Stewart, N. J., Parra-Robles, J., Collier, G. J. & Wild, J. M. Whole lung morphometry with 3D multiple b-value hyperpolarized gas MRI and compressed sensing. Magn Reson Med, doi:10.1002/mrm.26279 (2016).
6 Haacke, E. M., Lindskogj, E. D. & Lin, W. A fast, iterative, partial-fourier technique capable of local phase recovery. Journal of Magnetic Resonance (1969) 92, 126-145, doi:http://dx.doi.org/10.1016/0022-2364(91)90253-P (1991).
7 Lustig, M., Donoho, D. & Pauly, J. M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magnetic Resonance in Medicine 58, 1182-1195, doi:10.1002/mrm.21391 (2007).
8 Berberan-Santos, M. N., Bodunov, E. N. & Valeur, B. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential). Chemical Physics 315, 171-182, doi:10.1016/j.chemphys.2005.04.006 (2005).
9 Ouriadov, A., Lessard, E., McCormack, D. G. & Parraga, G. Can the Stretched Exponential Model of Gas Diffusion Provide Clinically -Relevant Parenchyma Measurments of Lung Disease? [abstract] ISMRM 24th Annual Meeting, 2788 (2016).
10 Parra-Robles, J., Marshall, H., Hartley, R. A., Brightling, C. E. & Wild, J. M. Quantification of lung microstructure in asthma using a 3He fractional diffusion approach [abstract]. ISMRM 22nd Annual Meeting, 3529 (2014).
Figures
Figure 1. Representative undersampled k-space masks. A) 50% under-sampled k-space in the phase-encoding direction, or acceleration factor equals two. B) 66% under-sampled k-space, or acceleration factor equals three.