1750
Exploring ex vivo brainstem complex pathways with diffusion microstructure at 11.7T1Center of NeuroImaging Research - CENIR, Paris, France, 2Inserm U 1127, CNRS UMR 7225, Sorbonne Universités, UPMC Univ Paris 06 UMR S 1127, Institut du Cerveau et de la Moelle épinière, ICM, Paris, France, 3AP-HP, Hôpital de la Pitié-Salpêtrière, Department of Neurosurgery, Paris, France, 4AP-HP, Hôpital de la Pitié-Salpêtrière, Department of Neurology, Paris, France
Synopsis
We performed multi-shell 3D segmented EPI on a post mortem macaque brain at 11.7T (resolution 200 μm iso, 120 directions) and evaluated NODDI as a tool to accurately extract brainstem pathways. We showed that the orientation dispersion map can help in segmenting ROIs used for tractography. Microstructure information opens promising perspectives for the exploration of the brainstem complex organization ex vivo in human and non-human primate.
Purpose
The brainstem is a region receiving lot of attention as a target for deep brain stimulation1,2. But its anatomical organization such as connectivity and spatial orientation of fibers tracts is not well known and needs further investigation. Diffusion MR microscopy has proven to be a useful tool to investigate the cytoarchitecture of brain tissues. Delimitation of brainstem pathways is uncertain due to overall lack of contrast, small region of interest, and ambiguous borders between nuclei and fiber tracts2. Recent models like neurite orientation dispersion and density imaging (NODDI)3 give the opportunity to probe the orientation dispersion. In this work, we evaluated NODDI as a tool to accurately extract brainstem pathways in macaque brain at 11.7T.Methods
Experiments were performed on a perfusion fixed macaque brain using a 11.7T Biospec 117/16 (Bruker Biospin, Germany) running Paravision 6.0.1 and equipped with a 72-mm transceiver. A 1:200 gadolinium MR contrast agent was added to the buffer 48 hours before imaging. Ex vivo diffusion imaging was acquired with a 3D segmented EPI sequence using 32 segments. Sequence parameters were: FOV 7.68*5.76*5.12 cm3; Mtx 384*288*256 leading to an isotropic resolution of 200 μm; TR/TE=250/26,6 ms; δ=6.5 ms and Δ=12.3 ms. Four different shells were acquired with b-values of 10 000, 4 000, 1 000 and 500 mm²/s with respectively 64, 32, 16, and 8 non-collinear directions. 9 A0 images (without diffusion gradient) were also acquired with the same parameters. Total scan time was 73 hours.
We fitted neurite orientation dispersion and density imaging (NODDI) using the AMICO Matlab toolbox4 : the intra-cellular volume fraction referring to the space bounded by the membranes of neurites, their orientation dispersion (OD), and the isotropic volume fraction.
Data were processed with MrTrix and we estimated the distribution of fiber orientations (FOD) present within each imaging voxel using the Constrained Spherical Deconvolution framework5. Probabilistic whole brain tractography was run with the following parameters: 100 000 tracks, step length of 0.1 mm, curvature threshold of 45°, minimum tract length of 6 mm, maximum tract length of 250 mm and fiber orientation distribution amplitude cut-off of 0.06. The spherical-deconvolution informed filtering of tractograms was applied to reduce the number of streamlines to 1 million to provide a biologically meaningful estimate of structural connection density by removing false positive tracts6. The tracts of interest in the resulting whole brain tractogram were isolated with manually defined regions of interest using the OD map.
Results
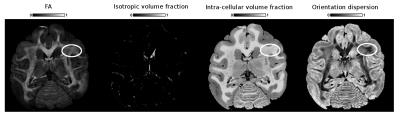
Example NODDI maps alongside the fractional anisotropy (FA) map are shown in Fig. 1 and are consistent with the known brain anatomy7. We observed that the intra-cellular volume fraction is lower in gray matter than in white matter contrary to the OD which is higher in gray matter than in white matter. The isotropic volume fraction is also coherent and takes its highest values for the expected CSF regions.
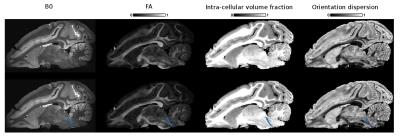
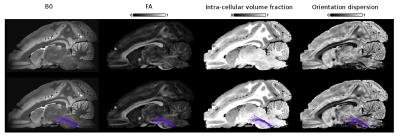
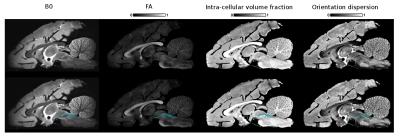
Figs 2, 3 and 4 show the reconstructed lateral lemniscus, medial lemniscus and medial longitudinal fasciculus pathways of the brainstem by segmenting ROIs on the OD map instead of the B0, FA or the intra-cellular fraction volume. Indeed, the OD map shows very clearly the tracks with a low orientation dispersion.
Conclusion
We showed that microstructure information, using the NODDI model, opens promising perspectives for the exploration of the brainstem complex organization ex-vivo. As NODDI is a clinically feasible in-vivo technique3, it could be valuable to extract brainstem pathways in patients undergoing deep brain stimulation of the mesencephalic locomotor region in order to improve targeting.Acknowledgements
The research leading to these results received funding from the programs 'Institut des neurosciences translationnelle' ANR-10-IAIHU-06 and 'Infrastructure d’avenir en Biologie Santé' ANR-11-INBS-0006.
SBS has been supported by the Bettencourt Schueller Foundation and by the National Agency for Research under the program "Investissements d’avenir" ANR-10-EQPX-15.
References
1. Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nature. 2007;8(8):623-35
2. Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, et al. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008; 131(Pt 6):1588–98.
3. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander D. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 20012; 61(4):1000-16
4. Daducci A, Canales-Rodriguez E, Zhang H, Dyrby T, Alexander D, Thiran JP. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. NeuroImage 105, pp. 32-44 (2015)
5. Dhollander T, Raffelt D, Connelly A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI, 2016, 5.
6. Smith RE, Tournier JD, Calamante E, Connelly A. SIFT: Spherical-deconvolution informed filtering of tractograms. NeuroImage. 2013;67:298-312.7. Zitella LM, Xiao Y, Teplitzky BA, Kastl DJ, Duchin Y, Baker KB, … Johnson MD. In Vivo 7T MRI of the Non-Human Primate Brainstem. Plos One. 2015, 10(5), e0127049.
Figures