1747
Reconstruction of fetal diffusion MRI using a spherical harmonic model1Division of Imaging Sciences & Biomedical Engineering, King's College London, London, United Kingdom, 2École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 3Health Services & Population Research, King's College London, London, United Kingdom
Synopsis
We present a novel method for reconstruction of fetal dMRI based on spherical harmonic model that includes motion correction, distortion correction and super-resolution reconstruction. We show that all these steps are important for producing good quality results. Our method will facilitate investigations into brain white-matter development in utero.
Purpose
Fetal diffusion MRI (dMRI)
offers the potential for obtaining detailed information about brain
connectivity and microstructure in-utero1. Reconstruction of
consistent fetal dMRI requires processing of data scattered in both the spatial
and angular domains caused by fetal motion, as well as correction of geometric
distortion that affects echo-planar imaging (EPI). Previous work employed
registration-based motion correction and scattered data interpolation2,3 or
super-resolution4 to reconstruct diffusion tensors. However, the diffusion
tensor model cannot fully describe complex connectivity structure, such as
fibre crossings. We therefore propose a novel method for reconstruction of
fetal dMRI from scattered data using a spherical harmonics (SH) model5,6,
which can be adapted for higher order analyses if supported by the acquired angular
resolution and b-value of the data. Additionally, we also perform correction of
susceptibility induced distortion7 and show that this results in
improved consistency of the diffusion data. The SH representation of diffusion
signal is estimated using a super-resolution approach, which is essential due
to thick-slice acquisitions that are common for fetal MRI.Methods
The single-shell diffusion weighted signal $$$s(\overrightarrow{g})$$$ varies with gradient sensitisation direction $$$\overrightarrow{g}$$$ and can be compactly represented using spherical harmonic basis functions5
$$s(\overrightarrow{g})=\sum_{lm}c_{lm}Y_{lm}(\overrightarrow{g})+n$$
where $$$c_{lm}$$$ are SH coefficients, $$$Y_{lm}$$$ are real SH basis functions of order $$$m$$$ and $$$n$$$ is Rician additive noise. Our aim is to reconstruct an estimated fetal dMRI signal $$$\overline{s}_i(\overrightarrow{g})$$$ in a SH representation on a high-resolution regular grid in anatomical space represented by index $$$i$$$. Low resolution acquired signals $$$s_{jk}$$$ of slice $$$k$$$ on in-plane grid represented by index $$$j$$$ can be estimated from this high resolution signal using convolution with the point spread function $$$m_{ij}^k$$$, which takes into account in-plane resolution, slice thickness, position and orientation of the fetal head in the scanner space at the time of acquisition8:
$$\overline{s}_{jk}(\overrightarrow{g}_k)=\sum_im_{ij}^k\overline{s}_i(\overrightarrow{g_k})$$
Each slice has a specific diffusion sensitisation direction $$$\overrightarrow{g_k}$$$ associated with it, which must take account of rotations introduced by fetal motion that may vary within each acquired volume2. The high resolution signal can be estimated from low-resolution acquired slices by minimizing the objective function $$$F(C)=\sum_{jk}(s_{jk}-\overline{s}_{jk}(\overrightarrow{g_k}))^2$$$ where $$$C$$$ represents set of all SH coefficients on the high resolution grid.
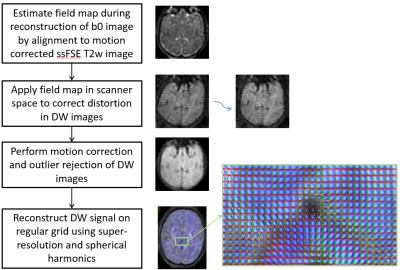
Diffusion MRI of seven fetal subjects (Gestational age of 24-33 weeks) were acquired using spin echo EPI (b=500 smm2, 15 directions, TE 121ms, TR 8500ms, FoV 290x290x128mm3, voxel size 2.3x2.3x3.5mm3, slice overlap 1.75mm). Spatial distortion was corrected using a field map estimated by maximising correspondence of b=0 images and ssFSE T2w images of the same subject using our previously proposed method7. Motion and outliers were estimated by co-aligning all slices of dMRI b=500 irrespective of the diffusion directions3 using our slice to volume reconstruction method8 originally proposed for structural fetal images. The high resolution SH representation of diffusion signal was then estimated by minimizing objective function $$$F(C)$$$ using gradient descent optimisation. Although the framework can support higher order spherical harmonics, in this study a second order model was used due to small number of diffusion directions available. The processing pipeline is summarized in Fig. 1.
Results
The method was
evaluated by reconstructing a SH representation of all subjects using only 10
volumes followed by simulating slices to match the remaining 5 diffusion
weighted volumes and calculating root mean square error (RMSE) between the
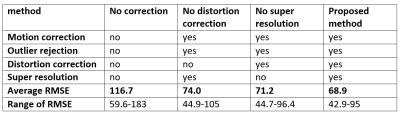
prediction and data. We evaluated effects of motion correction, distortion
correction and super-resolution. In the baseline comparison we did not perform
any correction and in all the others motion correction and outlier rejection
were performed. We compared the full proposed method to reconstruction where
either distortion correction was not performed or the thickness of the slice
was ignored and acquired data were considered to be isotropic. We found, that
motion correction, distortion correction and accounting for anisotropic PSF all
contributed to improvement of consistency of the data in all seven cases. The
average RMSE are presented in Fig. 2. In addition probabilistic tractography
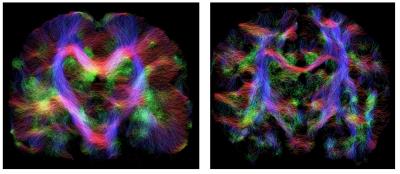
was performed using MRtrix6 operating on the full data sets,
producing results that match the expected anatomy in all cases (Fig. 3).Conclusion
We propose a spherical
harmonic model to reconstruct fetal dMRI from motion and distortion corrupted
EPI, and show that motion, distortion and voxel anisotropy all need to be
accounted for to produce good quality results. The method facilitates advanced in utero investigations into white
matter development.Acknowledgements
This work was supported by SNSF International Short Visit Grant (IZK0Z2_170894), MRC strategic funds (MR/K006355/1) and ERC funding - dHCP project (Grant Agreement no. 319456)References
1. Kasprian, G., et al., In utero tractography of fetal white matter development. Neuroimage, 2008. 43(2): p. 213-224.
2. Jiang, S.Z., et al., Diffusion Tensor Imaging (DTI) of the Brain in Moving Subjects: Application to In-Utero Fetal and Ex-Utero Studies. Magnetic Resonance in Medicine, 2009. 62(3): p. 645-65.
3. Oubel, E., et al., Reconstruction of scattered data in fetal diffusion MRI. Medical Image Analysis, 2012. 16(1): p. 28-37.
4. Fogtmann, M., et al., A Unified Approach to Diffusion Direction Sensitive Slice Registration and 3-D DTI Reconstruction From Moving Fetal Brain Anatomy. IEEE Transactions on Medical Imaging, 2014. 33(2): p. 272-289
5. Tournier, J.D., F. Calamante, and A. Connelly, Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage, 2007. 35(4): p. 1459-1472.
6. Tournier, J.D., F. Calamante, and A. Connelly, MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology, 2012. 22(1): p. 53-66.
7. M. Kuklisova-Murgasova, et al., Distortion correction in fetal EPI using non-rigid registration with Laplacian constraint, in IEEE International Symposium on Biomedical Imaging 2016: Prague.
8. Kuklisova-Murgasova, M., et al., Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Medical Image Analysis, 2012. 16(8): p. 1550-1564.