1742
White matter biomarkers from fast protocols using axially symmetric diffusion kurtosis imagingBrian Hansen1, Ahmad Raza Khan1, Noam Shemesh2, Torben Ellegaard Lund1, Ryan Sangill1, Leif Østergaard1, and Sune Nørhøj Jespersen1,3
1CFIN/MINDLab at the Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 2Champalimaud Neuroscience Programme, Champalimaud Centre for the Unknown, Lisbon, Portugal, 3Department of Physics and Astronomy, Aarhus University, Aarhus, Denmark
Synopsis
White matter tract integrity (WMTI) can be used to characterize tissue microstructure in areas with strongly aligned fiber bundles. Several WMTI biomarkers have now been validated against microscopy and provided promising results in studies of brain development, aging, and brain disorders. In clinical settings, however, the diffusion kurtosis imaging (DKI) protocol utilized as part of WMTI imaging may be prohibitively long. Consequently, the diagnostic value of the WMTI parameters is mostly explored in dedicated animal studies and clinical studies of slowly progressing diseases. Here, we evaluate WMTI based on recently introduced axisymmetric DKI which has lower data demand than conventional DKI.
Introduction
White matter tract integrity (WMTI) is used to characterize tissue microstructure in areas of aligned fiber bundles, Several WMTI biomarkers have been validated against microscopy and provided promising results in studies of brain development, aging, and disorders. In clinical settings, however, the diffusion kurtosis imaging (DKI) protocol utilized as part of WMTI imaging may be prohibitively long. Here, we evaluate WMTI based on recently introduced axisymmetric DKI with lower data demand than conventional DKI[3].Methods
Imaging was performed with a twice refocused spin echo DW EPI sequence with
inversion recovery[1] on a Siemens Trio 3T with a 32 channel head coil. One b=0 image and 33
directions on 14 b-value shells between 0.2-3.0 ms/µm2 were acquired,
with the directions a combination of a 24 point design (36) and the 9
directions for fast DKI [2, 3]. The effective diffusion time was approximately 50 ms. 19 consecutive
slices were acquired at 2.5 mm isotropic resolution with TR=7200 ms, TE=116 ms,
yielding an $$$SNR\approx 39$$$ at b=0. Conventional WMTI was implemented as [4]. Analytical WMTI was implemented by assuming axial symmetry in both the
extracellular and axonal compartments, enabling estimation of mean, axial,
radial kurtosis and diffusivity from only 19 images[3, 5]. All five WMTI parameters can then be expressed in terms of diffusion
kurtosis metrics:
$$
{{D}_{\bot }}=(1-f){{D}_{e,\bot }} $$
$$
{{D}_{||}}=f{{D}_{a,||}}+(1-f){{D}_{e,||}} $$
$$ {{W}_{\bot
}}{{{\bar{D}}}^{2}}=3f(1-f){{D}_{e,\bot }}^{2} $$
$$
{{W}_{||}}{{{\bar{D}}}^{2}}=3f(1-f){{({{D}_{a,||}}-{{D}_{e,||}})}^{2}} $$
$$\bar{W}{{{\bar{D}}}^{2}}=3f(1-f)\left[{{D}_{e,\bot}}^{2}+\frac{1}{15}({{D}_{e,||}}-{{D}_{a,||}}-{{D}_{e,\bot
}})\left( 7{{D}_{e,\bot }}+3({{D}_{e,||}}-{{D}_{a,||}}) \right) \right] $$
Two solutions exist, corresponding to two branches of the square roots [6, 7]. Only data in appropriate regions fulfilling the assumptions of WMTI
was analyzed, dictated by the Westin parameters as in [8].Simulations
The experimental signal from 100 random suitable WM voxels were fit with nonlinear least squares to the biexponential model underlying WMTI (no alignment or symmetries imposed). Parameter values obtained from the fit serve as input to the biexponential model to generate synthetic data with Rician noise (1000 noise realizations per voxel), $$$SNR=39$$$, matching the experimental data. The sampling scheme used for data acquisition was used in simulations, but with b-values between b=0-2.6 ms/µm2 . The synthetic signals were then analyzed in the same manner as the experimental data to yield WMTI parameters from conventional and analytical axisymmetric WMTI (aWMTI). A 1-9-9 subset of the simulated data with b1 = 1.0 ms/µm2 and b2 = 2.6 ms/µm2 was used to assess the WMTI estimates based on 1-9-9 data, referred to as analytical fast axial WMTI (faWMTI).Results
Figure 1 shows histograms of ground truth deviation in WMTI parameters from simulations corresponding to the correct branch (in this case Branch 1, $$$D_{a}< D_{e,||}$$$). Figures 2-4 show results from human brain in suitable WM voxels. Conventional estimates and aWMTI estimates from a full data set are shown in Fig. 2 for both branches. In Fig. 3, scatterplots compare the output of aWMTI from a full data set and a 1-9-9 data set for both branches. Finally, figure 4 shows histograms of WMTI diffusion parameters (both branches) from all suitable voxels.Discussion
The simulations indicated that aWMTI performs at least as well
as conventional WMTI when all b-values/directions were included (Fig. 1).
Consistent with this, WMTI metrics from both methods showed a high correlation
for both branches in the real data: the best agreement was found for $$$D_{e,\bot}$$$
(correlation coefficient 0.98), and the worst for intra-axonal diffusivity $$$D_{a}$$$
(correlation coefficient 0.81/0.69, branch 1/branch 2). When the data was
reduced to the 1-9-9 protocol (Fig. 3), agreement decreased, although remaining
high. Branch 1 showed the highest correspondence for extracellular parallel
diffusivity (0.81) and the lowest for the intra-axonal diffusivity $$$D_{a}$$$ (0.75),
while branch 2 showed the lowest correlation coefficient of 0.63 for $$$D_{a}$$$,
and the highest for extracellular axial diffusivity (0.76). The histograms of
human WM $$$D_{a}$$$ and $$$D_{e,||}$$$ values (Fig. 4) show the behaviors for
the 2 branches from WMTI (full data set). Notably, branch 2 had most
intra-axonal diffusivity values above 3 µm2/ms, lending support to branch
1 with $$$D_{a}< D_{e,||}$$$ as the
appropriate choice for white matter in vivo. Conclusion
Simulations indicated that WMTI parameters derived analytically from axially symmetric DKI are at least as accurate as conventional WMTI, and remain reliable when diffusion weighted images are reduced from 462 to 19. Analysis of histograms of white matter intra-axonal diffusivities preferred one branch over the other, with $$$D_{a}< D_{e,||}$$$. The results (especially reduced data requirements) may facilitate the application of the WMTI technique into a wider range of settings, including preclinical research and the clinical evaluation of patients who suffered traumatic WM injuries.Acknowledgements
Danish Ministry of Science, Technology and Innovation’s University Investment Grant (MINDLab, Grant no. 0601-01354B), and NIH 1R01EB012874-01, Lundbeck Foundation R83-A7548 and Simon Fougner Hartmans Familiefond. Lippert’s Foundation and Korning’s Foundation for financial support. Danish Research Council's Infrastructure program, the Velux Foundations, and the Department of Clinical Medicine, AU.References
1. Jones, D.K., et al., Neuroimage, 2013. 73. 2. Hansen, B., et al., Magn Reson Med, 2013. 69(6). 3. Hansen, B., et al., NeuroImage, 2016. 4. Fieremans, E., et al., Neuroimage, 2011. 58(1). 5. Hansen, B., et al. (2016) White matter biomarkers from fast protocols using axially symmetric diffusion kurtosis imaging. ArXiv e-prints 1610. 6. Jelescu, I.O., et al., NMR in biomedicine, 2016. 29(1). 7. Novikov, D.S., et al. (2016) ArXiv e-prints 1609.09144v1 8. Westin, C., et al., MICCAI'99. (1999) pp. 441-452Figures

Results from numerical simulations comparing performance
of WMTI to analytical axisymmetric WMTI. Please consult the methods for
simulation details. Histograms show relative errors for WMTI parameters for
each analysis method. The red line marks zero error. Text inside each plot
reports median relative error and 95%-inter-quartile range for each method.
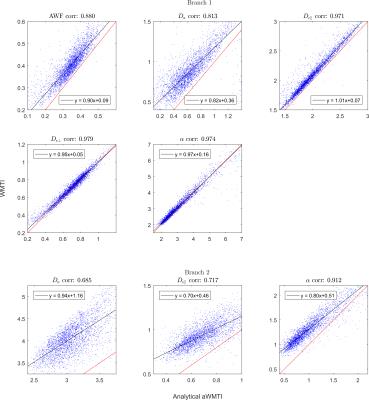
Comparison of parameter estimates in human WM
from WMTI and analytical axisymmetric WMTI (aWMTI). The top five panels show
branch 1 and the bottom three panels compare estimates in branch 2. $$$D_{e,\bot}$$$ and axonal water fraction have the same values in the 2 branches.
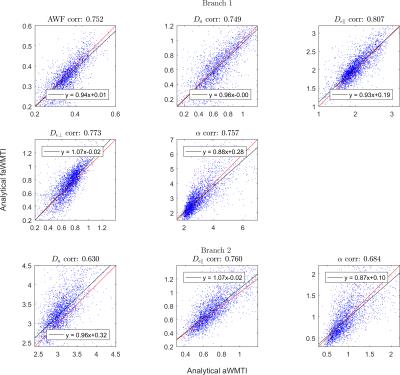
Comparison of parameter estimates in human WM
from analytical axisymmetric WMTI (aWMTI) and analytical estimates based on
data from the 1-9-9 protocol allowing fast axisymmetric WMTI (Analytical
faWMTI). The top five panels show branch 1 and the bottom three panels compare
estimates in branch 2. $$$D_{e,\bot}$$$ and axonal water fraction have the same values in the 2 branches.
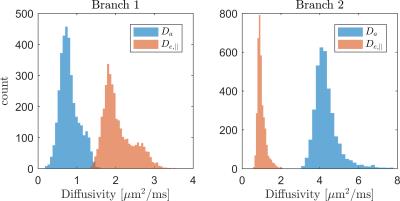
Histograms of
WMTI estimates of parallel extra- and intra-axonal diffusivities in
human WM for both branches.