1729
Functional magnetic resonance imaging shows network activation from a non-visual light stimulus of deep brain photoreceptors in songbirds1Department of Animal Science, The Pennsylvania State University, University Park, PA, United States, 2Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA, United States, 3Huck Institutes of the Life Sciences, The Pennsylvania State University, 4The Neuroscience Program, The Huck Institutes of Life Sciences, 5Interdepartmental Graduate Program in Neuroscience
Synopsis
Many avian species go through circannual changes in reproductive organs. Seasonal changes are triggered by non-visual photostimulation, made possible by the existence of nuclei with containing opsin channels. Photoactivation occurs when photosensitive neurons are stimulated, resulting in a cascade of activity in the brain, which initiates gonadal growth. Photorefraction is on a time-scale much longer than an experiment. We used echo planar imaging fMRI with a laser for stimulation and performed independent component analysis on each phase (before, during, and after stimulation). Network activation patterns differ in each phase.
Introduction
Seasonal breeding birds regulate their reproductive axis in response to changes in day length1,2. Photostimulation can occur only at certain times during the day. In winter the reproductive axis is not activated because light does not coincide with the photoinducible phase. When daylight coincides with photoinducibility, the reproductive axis activates. At the end of the reproductive phase, a period of photorefractoriness occurs in which the bird experiences gonadal regression and an inability of light to induce gonadal activation, even under stimulating conditions. This photorefractoriness is broken by placing birds in short days for several weeks. The neural centers controlling photostimulation and photorefractoriness are still unknown.
Birds have deep brain photoreceptors (DBPs) that are
responsible for day-length perception. The mechanism by which
the hypothalamus becomes insensitive to light appears to involve processes within the mediobasal hypothalamus3. Four different sites within the hypothalamus have shown to be potential
DBPs: the lateral septal area4, the paraventricular region5, the premammillary nucleus6, and the
paraventricular organ7. These sites were determined
by expression of antibodies against multiple specific opsins. It is not known why multiple individual
locations would be photoreceptive and why multiple opsin types are needed to
respond to photic information from the environment. Recent work by Kuenzel et
al.8 suggests that the different photoreceptor arrays may mediate
different aspects of the photostimulatory and photoinhibitory effects of light
on the reproductive axis. However, it is not known how neuronal pathways and
the individual nuclei within the mediobasal hypothalamus respond differentially
to photostimulation. We used functional magnetic resonance imaging (fMRI) to identify changes in activation networks while photostimulating deepbrain photoreceptors of photoinducible songbirds.
Methods
Animal Preparation and Image Acquisition
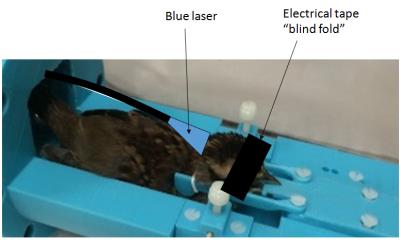
White-throated sparrows (Zonotricia albicollis) were taken from the aviary at night during lights out and brought to the scanner location in a light-proof box. All sources of light in the preparation room were blocked. Animal preparation was done under dim red light. Birds were initially anesthetized with 2.5%isoflurane in air, given a 30µl bolus of 50mg/ml followed by continuous 167nl/min infusion of Dexmedetomidine in 1%NaCl. Skull feathers were removed, birds were placed in a custom 3D printed holder (see Figure 1), and the eyes were covered with black electrical tape. A fiber optic cable was placed behind the bird's head such that laser beam spread covered most of the back head. Once in the scanner, isoflurane was turned off, while airflow was unchanged. A small animal monitor was used to track the respiration while in the scanner. High resolution T2-weighted structural images (TE=6.6ms, TR=4s, resolution=78x78x500µm2, averages=8, RARE factor=8, scan time=10m40s) were acquired on which to overlay functional activation. We used a multi-shot echo planar imaging (EPI) imaging sequence for BOLD fMRI experiments (TE=8ms, TR=750ms, Segments=2, resolution=312x312x700µm2, volumes=1600, scan time=40m). Initially, no stimulus was applied. After 10 minutes (400 volumes), a blue (420nm) laser continuously illuminated the birds skull for 20 minutes (800 volumes). The laser was then shut off and the animal scan continued 10 minutes. Following the experiments, a bolus of Atipamezole at 2-4x the dose of the Dexmedetomidine was given and the bird kept in a warming blanket until recovered.
Image processing
High resolution T2-weighted images and EPI image
sequences were masked using Avizo, and brain extracted using FSL. SPM was used to
corregister all the T2-weighted images to a
template and apply the transformation to the corresponding EPI image sequences. The images
were then smoothed in SPM with a .5mm Gaussian kernel. Group analysis was performed in GIFT (Group ICA of fMRI Toolkit) to find regions of coactivation. Because of the irreversibility of stimulation the data was divided into three separate epochs - pre-stimulation, during stimulation, and post-stimulation. GIFT was then set to find 20 independent components for each epoch.
Results and Discussion
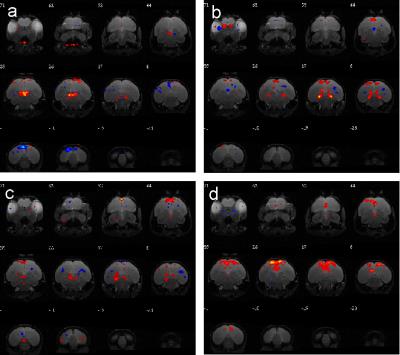
N=6 birds held in 6:18 light:dark cycle were scanned at night. The peak photoinducible time is ZT14 (Zeitgeber time, or time from lights on), so the EPI sequence was started 10 minutes before ZT14 and continue to 30 minutes after ZT14. The independent components of each state of stimulation were examined to determine functional networks. One resting state network was found during pre-stimulation. Three functional networks were found during stimulation. Four functional networks were found during post-stimulation (see Figure 2). Because of the irreversible nature of the photoactivation, it can not be determined which nuclei are responsible for activating these networks. Furthermore, it remains to be seen how these networks compare to stimulation of photorefractory birds. Nevertheless, to our knowledge, this is the first work showing functional activation of deepbrain photoreceptors in birds.Acknowledgements
This work was supported by the Huck Institutes of the Life Sciences to Bruce Langford and the Office of Naval Research Awards N00014-16-1-2187 and N00014-14-1-0703 to Paul Bartell.References
1. Farner, DS, Follett, BK. J. Anim. Sci. 1966 25, 90–118.
2. Kumar V, et al. Physiol Biochem Zool. 2010 83:827-35.
3. Dawson A, et al. J Biol Rhythms. 2001 Aug;16(4):365-80.
4. Silver R, et al. Cell Tissue Res. 1988 Jul;253(1):189-98.
5. Soni BG, Foster RG. FEBS Lett. 1997 Apr 14;406(3):279-83.
6. Kang SW, et al. Neuroscience. 2007 Nov 30;150(1):223-33.
7. Nakane Y, Yoshimura T. Front Neurosci. 2014 doi: 10.3389/fnins.2014.00115.
8. Kuenzel WJ, Kang SW, Zhou ZJ. Poult Sci. 2014 pii: PS4370.
Figures