1727
Relationship between GABA and glutamate and inter- and intra-regional intrinsic connectivity as measured by resting-state fMRI1Radiological Sciences, Division of Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom, 2Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 3Department of Radiotherapy and Radiation Oncology, OncoRay National Center for Radiation Research in Oncology, Dresden, Germany, 4Departments of Psychiatry and Medical Biophysics & Robarts Research Institute, Western University, London, ON, Canada, 5Lawson Health Research Institute, London, ON
Synopsis
Associations between GABAergic and glutamatergic systems and measures of brain connectivity have been reported previously. However, there is currently no evidence for associations between these systems and local connectivity measures, limiting our understanding of inhibitory/excitatory balance and connectivity across differing spatial ranges. We demonstrated that only regional connectivity and spontaneous activity, but not long-range connectivity, were associated with GABA and GABA/Glx ratio in the ACC. We suggest that this correlation between measures of intra-regional connectivity and GABA in the ACC may be driven by interneuron activity. Further, associations between neurotransmitter pools and inter-regional connectivity may be region/network and context-specific.
Introduction
The exact physiological and molecular mechanisms underlying functional activity in the human brain remains equivocal, though multimodal imaging has become highly informative in recent years, demonstrating the central role of GABA and glutamate in the inhibitory-excitatory balance that underlies neural activity1. Previously, associations between GABAergic and glutamatergic systems and resting-state fMRI-derived measures of brain connectivity have been reported2,3, though not consistently. One study has also linked degree of glucose metabolism and GABAergic function with regional connectivity measures at rest4. However, there is currently no evidence for associations between GABA and glutamate and local connectivity measures, which limits our understanding of inhibitory/excitatory balance and connectivity across differing spatial ranges. In the current study, we investigated the relationship between Glx, GABA and GABA/Glx ratio as measured by MRS, and local (regional homogeneity [ReHo] and fractional amplitude of low frequency fluctuations [fALFF]) and long-range functional connectivity (FC) measures using resting-state fMRI.Methods
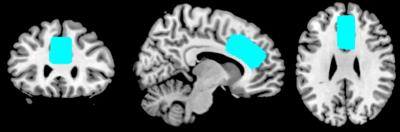
Twenty-eight healthy volunteers underwent resting-state fMRI and MR spectroscopy scans on a GE 3 Tesla MR750 system with a 32 channel head coil. Anatomical T1-weighted images were acquired using a 3D fast spoiled gradient echo (FSPGR) sequence acquired in sagittal orientation and 1mm isotropic voxel size (field of view (FOV)=256×256×156, repetition time (TR)=8.156 ms, echo time (TE)=3.172 ms, inversion time (TI)=900 ms). Whole-brain resting state functional images were acquired using an echo planar imaging sequence for a duration of 5 min 20 s with eyes open (TR=2 s, TE=32 ms, flip angle=90°, matrix size=64×64 mm, 160 volumes, 35 axial slices, 3.75×3.75×3.6 mm resolution). Single voxel 1H MR spectra were acquired using a PRESS sequence and optimized TE and TE1 for GABA detection with minimal macromolecule contamination5 (TR=2.5 s, TE=105 ms, TE1=15 ms, 128 averages, 16 water unsuppressed averages, 8 phase cycles), with the voxel placed in the anterior cingulate cortex (ACC) (30 x 20 x 15 mm; Fig 1). Following preprocessing of the resting-state fMRI data, spatial maps for ReHo and fALFF were calculated and entered into a voxel-wise regression analysis to look for associations with GABA, Glx and GABA/Glx ratio using small volume correction to constrain the search to within the MRS voxel. To explore whether GABA was related to long-range network inhibition, mean FC values were derived from regions showing negative and positive causal influence from the ACC voxel and correlated with GABA and Glx respectively. In addition, mean values for the default mode network (DMN) were extracted using independent components analysis and correlated with MRS measures.Results
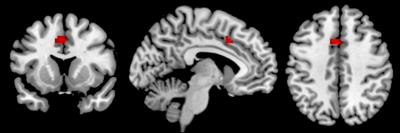
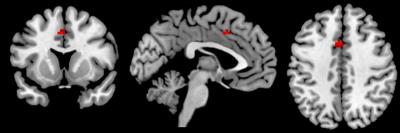
Significant negative associations were found for both ReHo and fALFF and GABA in the ACC (p<.05, FWE-corrected; Fig 2&3). In addition, ReHo was also significantly related to GABA/Glx ratio in the ACC. No long-range connectivity measures of FC and DMN connectivity were found to significantly associate with GABA or Glx.Discussion
Our study demonstrated that only regional connectivity and spontaneous activity indexed as amplitude of the low frequency fluctuations were associated with GABA levels and the GABA/Glx ratio. If GABA is primarily from interneurons, this is consistent with the notion of lateral inhibition whereby neighbouring pyramidal cells receive feedback inhibitory signals from interneurons. This is expected to result in reduced local activity with associated lower low frequency fluctuations and in reduced local synchronisation. Contrary to a previous study showing negative correlation between intrinsic DMN connectivity and GABA3, we did not find any links between neurochemistry and long-range connectivity measures. This may be due to regional differences in GABAergic modulation of inhibitory circuits with a lesser GABAergic effect from anterior DMN neurons compared to posterior DMN. The large voxel size also precludes to sample from ACC subregions that may exert GABAergic network inhibition with well documented subregional variation in GABA receptor density and spectroscopically determined GABA levels6. Lastly, this study only included young healthy controls in resting condition and GABAergic long-range network modulation might depend on baseline GABA and network activity levels.Conclusion
Measures of intra-regional connectivity and activity correlate with GABA in the ACC and may be driven by interneuron activity. However, associations between neurotransmitter pools and inter-regional connectivity may be region/network and context-specific.Acknowledgements
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement No PCOFUND-GA-2012-600181.References
1. Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—a review of multimodal imaging studies. Neurosci. Biobehav. Rev. 2014;47:36–52.
2. Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, Doyon J, Northoff G. Glutamate Concentration in the Medial Prefrontal Cortex Predicts Resting-State Cortical-Subcortical Functional Connectivity in Humans. PLOS ONE 2013;8:e60312.
3. Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. NeuroImage 2013;64:112–119.
4. Nugent AC, Martinez A, D’Alfonso A, Zarate CA, Theodore WH. The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J. Cereb. Blood Flow Metab. 2015;35:583–591.
5. Napolitano A, Kockenberger W, Auer DP. Reliable gamma aminobutyric acid measurement using optimized PRESS at 3 T. Magn. Reson. Med. 2013;69:1528–1533.
6. Dou W, Palomero-Gallagher N, van Tol M-J, Kaufmann J, Zhong K, Bernstein H-G, Heinze H-J, Speck O, Walter M. Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:12698–12704.