1723
Network Analysis Revealed Two Distinct Neuronal Circuits Involved in Nociceptive Processing within the Rat Brain after Ablation of TRPV1-Expressing Peripheral Neurons1Institute of Pharmacology and Toxicology, University of Erlangen-Nuremberg, Erlangen, Germany
Synopsis
Pain is a vital danger signal, as it prevents the body from suffering burns by hot items. Using BOLD-fMRI, we evaluated the rat's brain response to increasing thermal stimuli to detect which brain structures are involved especially in the processing of nociceptive heat. Therefore, we eradicated selectively the nociceptive-specific TRPV1-expressing neurons via Resiniferatoxin-induced excitotoxicity. This missing nociceptive input from the periphery resulted in a widespread suppression of brain activity in most brain structures except some parts of the brainstem. Graphtheoretical network analysis revealed two distinct circuits of brain structures involved in the processing of noxious temperatures above 48°C.
Introduction
Resiniferatoxin (RTX), an extract from a plant of the spurge family, Euphorbia resinifera, is a potent agonist of the transient receptor potential cation channel subfamily V member 1 (TRPV1). TRPV1 is mainly expressed on small-diameter unmyelinated peripheral C-fibers, where it serves as a multimodal nociceptor. Beside endogenous ligands and protons, the channel pore opens up with temperatures above 43°C, mediating the life-saving avoidance response to noxious heat.
Administered peripherally in high doses, RTX induces calcium-mediated excitotoxicity in TRPV1-expressing neurons and can therefore be used to ablate heat nociceptors with high specificity. Aim of this study was a) to characterize the central processing of innocuous and noxious heat stimuli, b) to define a threshold between warmth and heat sensation in rats and c) to identify brain structures that are critically involved in the processing of noxious heat by BOLD fMRI.
Material and Methods
Male Wistar rats (~ 400g) were separated into two groups, one remained naïve (n=18) while the other one was treated with RTX (n=9) [1]. RTX was injected subcutaneously into the neck skin on 3 consecutive days with increasing doses (30, 70, 100µg/kg) (for treatment of side effects such as respiratory problems see [2]).
FMRI BOLD-measurements (4.7T Bruker Biospec, 2 x 2 array head coil, matrix 64 × 64, FOV 25 × 25mm, voxel size 391 x 391µm, slice thickness 1mm, axial, 22 slices, GE single-shot EPI (TR = 4000 ms, TEef = 24.38 ms)) were conducted under isoflurane anesthesia 8 days after the last injection.
To monitor brain responses closely, we applied a contact heat stimuli sequence that ranged from 40°C up to 54°C in 2-degree-steps. To avoid anticipation effects, the stimuli were presented in a pseudo-randomized fashion at the left hind paw (duration 20sec, 3min and 40sec interval; each temperature repeated three times in total; actively feedback computer-controlled Peltier heating device).
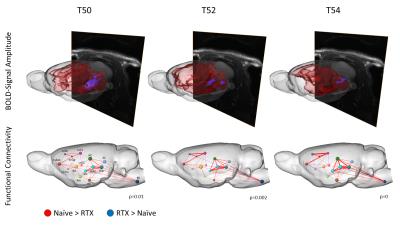
First of all, data was analyzed using classical BOLD-analysis approach implying quantification of activated brain volume and activation strength for functionally connected groups of brain structures. Secondly, because results of BOLD-analysis were significant, but too imprecise in the sense of structure specificity and information flow, we applied graphtheoretical network analysis and calculated differences in functional connectivity of 202 distinguishable brain structures between both experimental groups. Significance of detected differences was statistically verified using network based statistic, a novel statistical method to control the family-wise error rate of mass-univariate comparisons [3].
Results
Ablation of TRPV1-expressing neurons via RTX suppressed effectively the BOLD brain response and reduced the activated brain volume evoked by thermal stimuli above 46°C in almost all brain regions with exception of the pons, tegmentum and the Nucleus gigantocellularis, which showed greater activation in RTX-treated animals. These structures are involved in respiratory control, a finding that fits the previously described respiratory problems due to an enhanced mucus secretion following RTX injection, and might implicate that those are sustained effects [2].
For temperatures below 48°C, the BOLD brain response was comparable between both groups, indicating that 48°C skin temperature (which differs from local temperature at the nociceptor) can be described as the nociceptive heat threshold for rats.
Applying sophisticated graphtheoretical network analysis, we found a significant reduction of functional connectivity within two rather distinct circuits of brain structures. Functional connectivity serves as a measure to describe the interaction and information flow between brain structures. We found differences to be significant only for temperatures above 48°C, and disconnectivity correlated positively with increase of temperature. The first circuit consisted of the thalamus, the hippocampus, the superior colliculi and the brainstem, while the other one contained the primary somatosensory cortex, motor cortex, association cortex and the cingulum. Those two circuits are interlocked via thalamo-cortical and hippocampal-cortical projections, which function alike processing innocuous or noxious temperatures as they show no difference in connectivity between both groups.
Conclusion
Missing nociceptive input from the periphery due to ablation of TRPV1-expressing neurons via RTX leads to a widespread reduction of brain activity evoked by noxious heat stimuli, as one would expect. Via graphtheoretical network analysis, we were able to distinguish two affected brain circuits (thalamus-hippocampus and cortical structures) which are involved in naïve animals in filtering and integrative processing of nociceptive signals specifically, as well as in sensory localization and emotional interpretation. Interestingly, thalamo-cortical as well as hippocampal-cortical connections remained unaffected, indicating that they are involved in the processing of any kind of thermal stimuli, independent of their magnitude.Acknowledgements
BMBF NEUROIMPA (2015-2019) TP4 01EC1403CReferences
[1] Mitchell K, Lebovitz EE, Keller JM, Mannes AJ, Nemenov MI, Iadarola MJ (2014). Nociception and inflammatory hyperalgesia evaluated in rodents using infrared laser stimulation after Trpv1 gene knockout or resiniferatoxin lesion. Pain 155(4): 733-745.
[2] Karmouty-Quintana H, Cannet C, Sugar R, Fozard JR, Page CP, Beckmann N (2007). Capsaicin-induced mucus secretion in rat airways assessed in vivo and non-invasively by magnetic resonance imaging. British journal of pharmacology 150(8): 1022-1030.
[3] Zalesky A, Fornito A, Bullmore ET (2010). Network-based statistic: identifying differences in brain networks. NeuroImage 53(4): 1197-1207.
Figures