1722
Functional Connection Between Brain and Right Upper-arm: A rs-fMRI Study1Department of radiology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan, 2Department of Electrical Engineering, National Taiwan University, Taipei, Taiwan, 3Neurobiology and Cognitive Science Center, National Taiwan University, Taipei, Taiwan, 4Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
Synopsis
This study aimed to explore the functional coupling between the central nervous system and the peripheral nervous system of upper limb. We established a novel imaging-platform to collect the functional images from human brain and upper limb simultaneously. This study disclosed the functional coupling between the peripheral nervous system of upper limb and the brain cortex and demonstrated that peripheral nervous system also involved in the regulation of brain cognitive function and related network changes.
Introduction
Task-free brain fMRI
possesses oscillations and complex networks across cerebral hemispheres and
specific areas (Achard, Salvador, Whitcher, Suckling, & Bullmore, 2006),
and this functional connectivity has strong relationship, although not
necessarily direct, with structural connectivity (Honey, et al., 2009). Because
axons extend outside the brain, if resting-state functional connectivity
depends on intact nerve fibers, BOLD correlations should also exist outside the
brain, for example, the limbs. And it should be different from the
corticomuscular coherence, which exists during active or pathological muscle
contraction (Mima & Hallett, 1999; Timmermann, Gross, Kircheis, Haussinger,
& Schnitzler, 2002). In this preliminary study, we tried to establish the imaging
platform to collect the echo planar imaging (EPI) signals under the
experimental design of task-free fMRI from human brain and upper limb
simultaneously. Methods
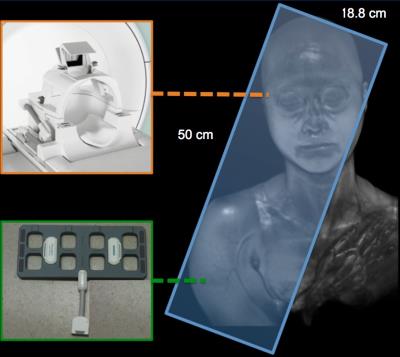
Task-performing and task-free functional data were applied to thirty-four participants (No.201402037RINB). Data from 3 participants were excluded due to motion issue. The experiments were performed with a 3T Siemens Prisma MR System (Siemens, Ettlingen, Germany). All data were acquired using one 20-channels head coil and one 18-channels flexible surface coil. The parameters of functional scans (EPI sequence) were used as follows: TR and TE were set at 3000 and 30 (ms); the in-plane field of view (FOV) was 500 mm x 500 mm; slice thickness was 4 mm without gap; matrix size was 128 x 128 x 47 to cover the whole brain and right upper arm (Figure 1). Participants were asked to open their eyes and do not think systematically. SPM12 software and REST toolbox were used to data preprocessing and analysis.Results
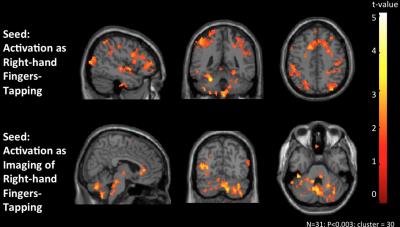
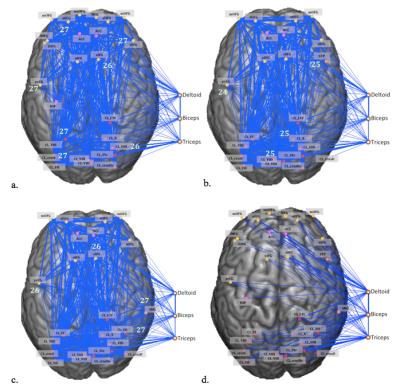
The peripheral activation during right-hand motor tasks was used as seed ROIs for the functional connectivity analysis based on task-free rs-fMRI data, and many networks were included in the functional connectivity maps as seeding at the peripheral active voxels (Figure 2.). In the rs-fMRI result, our preliminary result showed that the most-linked hub-regions in deltoid’s network included the precentral gyrus, superior OFG, inferior OFG, olfactory gyrus, cerebellar lobule VII, and bilateral cerebellar lobule VIII (Figure 3a); in the biceps’s network, the most-linked hub-regions included the precentral gyrus, middle temporal pole, cerebellar lobule VII, and cerebellar lobule X (Figure 3b); in the triceps’s network, the most-linked hub-regions included the precentral gyrus, olfactory cortex, inferior temporal gyrus, and cerebellar lobule VII (Figure 3c). We establish three networks which most-linked hub-regions in deltoid, biceps and triceps network. This preliminary could be one reference for the following brain-periphery connection and for the index of investigation.Conclusion
This study supported the notion that brain spontaneous oscillations might spread outside of the brain, possibly through its fibers, synapses, and neuromuscular junction, and these oscillations could be detected under task- free and non-motion period. In addition, upper limb also showed the functional synchronization with the default-mode network, and this connectivity might demonstrate that peripheral systems also join in the mental basis of central nerve system.Acknowledgements
This study was supported by grant projects (MOST 103-2321-B-002-097) from Ministry of Science and Technology in Taiwan. Thanks to the Instrumentation Center, National Taiwan University for MRI facility support.References
1. Achard, S., Salvador, R., Whitcher, B., Suckling, J., & Bullmore, E.(2006). Journal of Neuroscience, 26(1), 63-72.
2. Honey, C., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J., Meuli, R., et al. (2009). Proceedings of the National Academy of Sciences, 106(6), 2035-2040.
3. Mima, T., & Hallett, M. (1999). J Clin Neurophysiol., 16(6), 501-511.
4. Timmermann, L., Gross, J., Kircheis, G., Haussinger, D., & Schnitzler, A. (2002). Neurology, 58(2), 295-298.
Figures