1721
Modulation of Saslience Network Activation after 4 Weeks Verbal Training in Older Adults1NeuroImaging & Informatics, NCGG, Ohbu, Japan, 2Department of Radiological Science, Nagoya University Graduate School of Medicine, Nagoya, Japan, 3Faculty of Human Sciences, Kobe Shoin Women's University, Kobe, Japan, 4NeuroImaging & Informatics, National Center for Geriatrics & Gerontology, Ohbu, Japan, 5Department of Social and Human Environment, Graduate School of Environmental Studies, Nagoya, Japan, 6College of Liberal Arts and Sciences, Mie University, Tsu, Japan
Synopsis
The potential of RSN to detect the early change of neuronal networks induced by short-term cognitive intervention was investigated by using a verbal training task to read short sentences aloud everyday for 4 weeks. Twenty community dwelling older adults participated in this study. Activation in the anterior SN was decreased after the training, suggesting optimization of salience processing to integrate visual information and language production. The SN may be potentially a biomarker to firstly reflect the change in response to cognitive interventions in older adults and this finding may be applied to optimize training protocols for each individual.
Introduction
Resting state networks (RSNs), which are assumed to be the steady state activities of brain and prominent during resting status, have been studied for many purposes [1, 2]. In this study, we attempted to investigate the potential of RSN to detect the early change of neuronal networks induced by a short-term cognitive intervention. It will be useful for social programs to support cognitive functions of older adults, if we could predict the outcome of cognitive training 1 or 2 months after starting the intervention so that the program can be optimized. A verbal training model was employed for this purpose, since recording of behavioral data to evaluate the training performance is not difficult and motor function is strongly involved in speech production. It is known that speech articulation declines with age [3], however, it has not been much utilized as an index of cognitive intervention. Cognitive trainings not depending on the mobility of subjects will take one part in social programs to support older adults. The RSN status before and after four weeks of articulatory training was compared.Materials & Methods
Twenty healthy older adults (Age 61–76, 8 females), who gave written informed consent approved by the IRB, participated in this study. The training procedures were designed as the followings. Day 1st: Psychological tests (handedness, MMSE, GDS), and RS-fMRI. Day 2nd-28th: Training sessions (5 days per week, 4 weeks), Day 29th: RS-fMRI. On Day 1st, the subjects read aloud Japanese phrases which consisted of real and pseudo words with hard and easy consonants (20 sentences for each condition). They performed verbal training to read aloud the whole sentences 8 times as quickly, as accurately, and as loudly as possible for each sentence. Functional images were obtained by using an EPI sequence on a 3T MRI scanner (TR 3000ms, TE 30ms, FA 90deg, 39 axial slices, 3mm thick, 0.75mm interval, matrix 64x64, FOV 192mm, 140 volumes). The subjects were instructed to fix their eyes to a cross mark displayed on a LCD monitor and to keep rest for 7 minutes. After preprocessing of fMRI data sets using SPM12 (UCL. London), RSN activation was obtained by a group ICA toolbox (GIFT4.0, UNM, NM). Twenty-five independent components (ICs) were obtained by using Infomax algorithm with stability evaluation (bootstrap ICASSO), and the ICA was run for 20 times. A back reconstruction of each subjects’ contrast maps were performed to evaluate the effects of verbal training. The final contrast maps were obtained by a paired t-test (SPM12, k>10, p<0.001, uncorrected).Results
The 20 subjects could complete the 4 weeks' training according to the protocol. The MMSE scores were no less than 27 except one subject (24). ANOVA including the trained words at the post-training session revealed significant main effects of group (p < 0.05), training, and semantics, and all the interaction effects among the three factors (at p < 0.001). Contrast of decreased activation of RSN after the 4 weeks training was detected in the anterior salience network (SN)(T = 4.15, [4 28 26], BA32, anterior cingulate gyrus) (Figure.1, 2). No change was detected in dorsal / ventral default mode network (DMN) or in other RSNs.Discussion
Physical or cognitive tests to evaluate the outcomes of interventions do not have direct insights into their neuronal mechanisms. The roles of neuroimaging will be to explain the rationality of the interventions and supply a framework to optimize its protocol for each individual. For this purpose, RS-fMRI will be one useful tool, since it does not depend on particular tasks and comprehensively and systematically include principal neuronal networks [4]. In this study, activation in the anterior SN was partially decreased after 4 weeks’ verbal training. The SN is involved in identification of cognitively relevant events to guide flexible behavior in communication, social behavior, and self-awareness through the integration of sensory, emotional, and cognitive information [5]. In contrast to DMN, activation in the SN has been reported to augment in normal aging [6] suggesting more demand for perception of the surrounding environment. One possible explanation may be optimization of salience processing rather than its declination. The training task employed in this verbal training required integration of visual and language processing as well as attention to read the sentences fast, which will demand the role of SN. Since significant changes in other RSNs was not observed at this early time point, SN may be potentially a biomarker to firstly reflect change in response to cognitive interventions in older adults.Acknowledgements
This study was supported by the JSPS-NTU Research and Development grant, under the Japan-Singapore Research Corporative Program, FY 2014-2015, and by a Grant-in-Aid for Scientific Research (KAKENHI) # 24300186 and 15H03104 supported by MEXT.References
[1] Fox MD et al., The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PRONAS, vol.102, 9673-78, 2005 [2] Cole DM et al., Advances and pitfalls in the analysis and interpretation of resting-state FMRI data, Frontiers in System Neuroscience, vol.7, Article 9, 1-15, 2010 [3] Shafto MA. Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, vol.346, 583-587, 2014 [4] Shirer WR et al, Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, vol.22, 158-65, 2012 [5] Menon V, Salience network, in: Toga A.W. editor, Brain Mapping: An Encyclopedic Reference, Academic Press, vol.2, 597-611, 2015 [6] Chen SHA et al., Age-related Changes in Resting-State and Task-Activated Functional MRI Networks. IEEE Proceedings, Medical Information and Communication Technology (ISMICT), 218-222, 2013.Figures
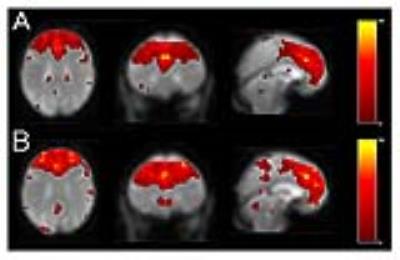
Figure 1
The IC of anterior SN before (A) and after the 4 weeks training (B) obtained from the 20 older subjects. After extracting ICs using GIFT4.0, a back reconstruction of each subjects’ contrast maps were performed to evaluate the effect of verbal training. The final contrast maps were obtained by a paired T-test (k>10, p<0.001, uncorrected) using the statistical tool of SPM12.
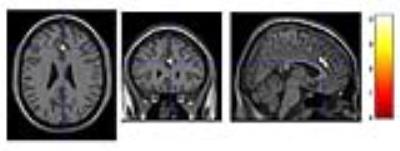
Figure 2
The contrast of pre-traning againt after-training of the IC of anterior SN (BA32, [4 28 26], T = 4.15, uncorrected). Activation in this area was decreased after 4 weeks of verbal training in older adults.