1717
Reorganization of Rodent Resting-state Functional Networks after mild Traumatic Brain Injury1Translational Imaging Research Center, College of Medicine, Taipei Medical University, Taipei, Taiwan, 2Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, 3Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 4Department of Medical Research, Taipei Medical University Hospital, Taipei, Taiwan, 5Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan, 6School of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan
Synopsis
Complex network analysis unraveled the mTBI-related functional reorganization with the disruption of long-distance connections of thalamocortical interactions and enhanced local connectivity segregation in the regions of neocortex and thalamic nuclei.
Background and Purpose
Mild traumatic brain injury (mTBI) is a complex disease with temporary or persistent cognitive and behavioral disturbance while the gross brain structures appear to be normal in the early phase. Recent studies revealed the abnormalities of functional connectivity after mTBI based on the resting-state fMRI (rsfMRI)1-3. However, it is still unclear how the intricate functional networks reorganize in response to the brain injury, and how the injury-induced functional reorganization can be correlated to the extent of mTBI. In this study, an experimental mTBI rodent model was employed to investigate the functional reorganization after injury. We anticipated the occurrence of altered functional connectivity related to the impact site, and aimed to investigate the corresponding network reorganization and its relations to the extent of injury. Exploratory network analysis based on the graph theory was employed to globally (whole-brain network) and locally (region-wise) assess the characteristics of functional network measured by the blood oxygenation level dependent (BOLD) rsfMRI.Materials and Methods
The animal study was approved by the Institutional Animal Care and Use Committee with the agreement of the 3Rs principle. Male Sprague–Dawley rats (weighing 250–400g) were anesthetized and placed in a stereotactic frame. An mTBI model modified from weight-drop impact acceleration injury4 with a weight of 600 gw dropping through a 1m-height tube into the metal disc cemented on top of the skull of the left primary somatosensory cortex was used. Two experimental conditions, a single impact (6 rats) and repetitive impacts with an interval of 1 hour (6 rats), was employed to create different mTBI extents. Animal MRI data were acquired using a Brucker 7T PharmaScan scanner with a circular surface coil for signal receiving. A RARE sequence (TR/TE=2650/40 ms; voxel size= 0.13x0.13x1.00 mm3) was performed to acquire anatomical images. BOLD rsfMRI was acquired using a single-shot GE-EPI (TR/TE=1000/15 ms; voxel size= 0.55x0.55x1.00 mm3; 300 volumes) under a combined anesthetic regime of low-dose isoflurane and dexmedetomidine infusion5. Physiological parameters were continuously monitored and maintained within normal ranges during the experiment. In total, data at pre-injury (n=12) and 24 h after injury (each n=6 after single and repetitive injury) were used in this study.
fMRI preprocessing steps included the correction of slice timing, realignment, co-registration between subjects, and spatial smoothing using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The gray matter was partitioned into 3 categories, including neocortex (NE, 10 regions), subcortical regions (SC, 12 regions), and thalamic nuclei (TN, 10 regions) for each hemisphere based on Waxholm Space atlas (Table 1)6,7. The Pearson cross-correlation coefficients were computed between regional BOLD signals (between 0.01-0.3 Hz)8 with regression of motion parameters followed by Fisher’s r-to-z transform. Topological parameters based on the graph theory were then applied to characterize functional networks9. One-sample t-test was employed to determine the significance of functional connectivity within a condition (p<0.05, with FDR correction), and two-sample t-test was used to determine the differences between single-/repetitive-impact and pre-injury condition (p<0.05).
Results and Discussion
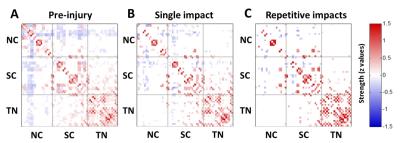
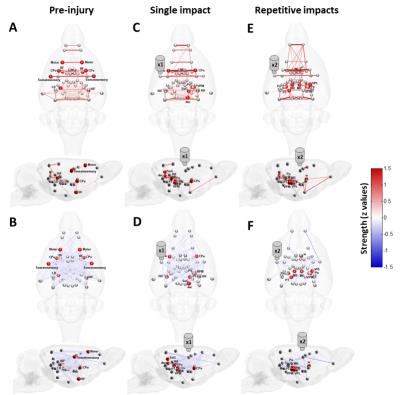
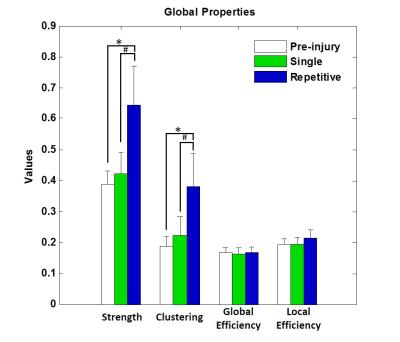
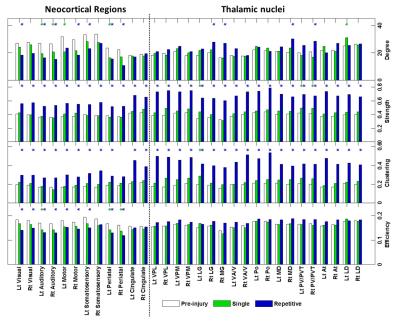
The applied animal model successfully mimicked the radiological traits of mTBI, i.e., no skull fracture and intact brain structure on RARE T2-weighted images. Persistent declines in neurological and general locomotor performance were observed in animals after injury. Our results of network analysis also revealed the alterations of functional organization. Substantial functional connections, with mostly positive connectivity for adjacent regions and negative values for long-distance connections between NC, SC, and TN, were presented on the connectivity matrix before injury (Fig.1A). When injury extent increased, the functional organization tended to lose the long-distance connections, especially between NC and TN (thalamocortical circuits) and elevated the connectivity strength within categories (Fig.1B and 1C). We further investigated the transitions of network hubs (pivotal regions with 15% top rank of connectivity strength) between conditions. Before injury, the hubs were located at bilateral somatosensory, motor, striatum, and basal forebrain regions (Fig.2A) and transferred to the TN regions, including sensorimotor-associated nuclei (VPM, VPL), mediodorsal nuclei (MD) and posterior nuclear group (Po) (Fig.2C and 2E). The global topological parameters of functional networks confirmed an impact-related increment of connectivity strength and clustering coefficient (local segregation) while preserving the network efficiency (Fig.3). Finally, the local topological parameters further revealed a significantly decreased degree (number of functionally connected neighbors) and efficiency at the impacted left somatosensory region (Fig.4). Several TN regions showed contrary increases of degree, larger increments of strength and clustering coefficient (Fig.4). Our results demonstrated a compensatory mechanism in mTBI involving the regulation of thalamocortical circuits presented by a functional reorganization after injury.Acknowledgements
This study was funded in part by the Taipei Medical University (TMU103-AE1-B20) and the Ministry of Science and Technology (MOST 105-2314-B-038-014, MOST 104-2923-B-038-003-MY3, MOST 105-2628-B-038-002-MY2), Taipei, Taiwan.References
1. Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Human brain mapping. 2011;32(11):1825-35.
2. Shumskaya E, Andriessen TM, Norris DG, Vos PE. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology. 2012;79(2):175-82.
3. Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, Grossman RI, Ge Y. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265(3):882-92.
4. Marmarou A, Foda MA, Brink WV, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats: Part I: Pathophysiology and biomechanics. Journal of neurosurgery. 1994;80(2):291-300.
5. Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proceedings of the National Academy of Sciences. 2012;109(10):3979-84.
6. Papp EA, Leergaard TB, Calabrese E, Johnson GA, Bjaalie JG. Waxholm Space atlas of the Sprague Dawley rat brain. NeuroImage. 2014;97:374-86.
7. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th ed. Academic Press, 2007.
8. Grandjean J, Schroeter A, Batata I, Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage. 2014;102:838-47.
9. Lu CF, Soong BW, Wu HM, Teng S, Wang PS, Wu YT. Disrupted cerebellar connectivity reduces whole-brain network efficiency in multiple system atrophy. Movement Disorders. 2013;28(3):362-9.
Figures