1714
Altered gray matter cerebral blood flow and its connectivity indicate a potential cognitive dysfunction of chronic subcortical stroke patients1Department of MRI, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People's Republic of China, 2Department of Radiology, Tianjin Key Laboratory of Functional Imaging, Tianjin Medical University General Hospital, Tianjin, People's Republic of China, 3School of Medical Imaging, Tianjin Medical University, Tianjin, People's Republic of China, 4GE Healthcare MR Research China, Beijing
Synopsis
In order to investigate gray matter cerebral blood flow (CBF) and CBF connectivity alterations in chronic stroke patients, 60 patients and 60 controls were recruited to undergo 3D ASL technique. The patients exhibited increased CBFs in contralesional SFG, thalamus and ITG, and decreased CBF in ipsilesional Post_CG. Further analysis showed decreased CBF connectivity in patients in ipsilesional Pre_CG, MFG and Msfg. Importantly, the patients exhibited disconnections between the SFG and MFG, mSFG, Pre_CG. Current results suggest that stroke-induced cognitive dysfunction may be a connectivity disorder from the perspective of CBF connectivity.
Purpose
To investigate the cerebral blood flow (CBF) alteration patterns and whether the CBF alteration as well as CBF connectivity changes in chronic subcortical stroke patients who were well recovered in global motor function.Methods
A total of 120 subjects were investigated to undergo imaging and behavioral tasks including 60 patients (F/M: 16/44, ages range: 40-75 years) making a remarkable recovery in global motor functional (Fugl-Meyer test score > 60 and whole extremity Fugl-Meyer test score > 90) with an unilateral ischemic infarct, involving the internal capsule and neighboring regions (Fig 1), and 60 healthy controls (F/M: 26/34, ages range: 40-75 years ) with matched age, gender and education level. The imaging data were acquired using GE Discovery 750 MR scanner.
1. The sagittal 3D T1-weighted images were acquired by a brain volume sequence: TR/TE = 8.2/3.2 ms; FOV = 256×256 mm2; matrix = 256×256; slice thickness = 1.0 mm, no gap; 188 slices. The perfusion imaging was performed by a 3D pcASL sequence: TR/TE = 5025/11.1ms; FOV =240×240mm2; post-label delay = 2025 ms; spiral in readout of 8 arms with 512 sample points; reconstruction matrix = 128; slice thickness = 3mm, no gap; 48 axial slices.
2. The CBF maps were derived from the ASL difference images that were calculated via the subtraction between label images and control images1. The individual CBF images were coregistered to the MNI-standard CBF template2. The CBF of each voxel was normalized by dividing the mean CBF of the whole brain3. The normalized CBF images were spatially smoothed with a Gaussian kernel of 8 × 8 × 8 mm3 FWHM.
3. Characterizing CBF concurrent changes across subjects between pairs of brain regions by computing the correlation coefficient is able to provide a CBF connectivity measure among these brain regions4. To test whether the brain regions with altered CBFs also had altered CBF connectivity in chronic subcortical stroke patients, the clusters with significant group differences in the CBF were selected as the seed ROIs. The CBF value of each ROI of each subject was extracted from an individual CBF map. For each group, multiple regression models were used to calculate the CBF connectivity between each seed ROI and all other voxels of the whole brain across individuals using age, gender and education level as confounding covariates. For each ROI, the CBF connectivity maps of the two groups were merged into a spatial mask where the CBF of each voxel was correlated with the CBF of the ROI in either of the two groups. To map the voxels that expressed a significantly different CBF correlation with each seed ROI between the patients and the healthy subjects, specific T contrasts were established within the spatial mask of the CBF connectivity map of the ROI. Multiple comparisons were corrected using a FWE method (p < 0.05).
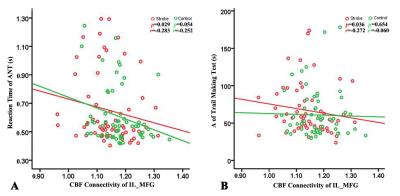
4. We performed Spearman correlation analysis to investigate the association between the clinical behavior scores and CBF connectivity.
Results
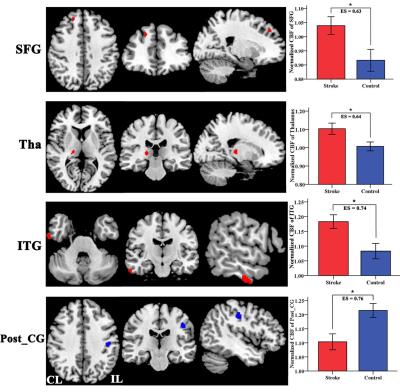
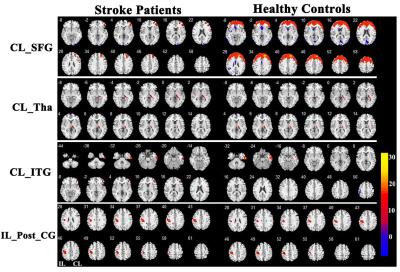
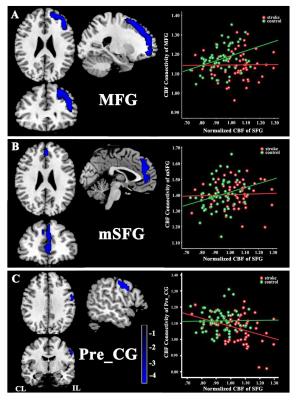
The stroke patients exhibited increased CBF in contralesional superior frontal gyrus (SFG) , thalamus (Tha) and inferior temporal gyrus (ITG), and decreased CBF in ipsilesional postcentral gyrus (Post_CG) (Fig.2). Further analysis showed that the SFG exhibited significantly different CBF connectivity pattern in both groups. However, the Tha, ITG and Post_CG had a similar connectivity pattern in both groups (Fig. 3). The patients showed decreased CBF connectivity in ipsilesional precentral gyrus (Pre_CG), medial frontal gyrus (MFG) and medial part of SFG (mSFG) (Fig.4). Importantly, the patients exhibited disconnections between the contralesional SFG and ipsilesional MFG, mSFG, and Pre_CG (Fig.4). Negative correlations between the decreased CBF connectivity and clinical parameters were shown in Fig.5.Discussion
The differences in normalized CBF signal and CBF connectivity under a strict threshold (FWE correction) between stroke patients and controls were explored using a 3D-pcASL technique. The present results demonstrated both regional CBF abnormalities and deficits in CBF connectivity in chronic subcortical stroke patients, which may further support the hypothesis that stroke-induced cognitive dysfunction after stroke is a connectivity disorder from the perspective of CBF connectivity.Conclusion
The study can identify diminished CBF connectivity in the cognitive network system in chronic stroke patients for the first time. It emphasizes the need for investigating the underlying mechanism of stroke-induced cognitive dysfunction in chronic subcortical stroke patients from the perspective of both regional and inter-regional properties of resting-state CBF.Acknowledgements
This study was supported by the Natural Science Foundation of China (Nos. 81601467, 81671659, 81401380).References
1. Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion mri on 3.0 t and comparison with 15o-water pet in elderly subjects at risk for alzheimer's disease. NMR Biomed. 2010;23:286-293.
2. Zhu J, Zhuo C, Qin W, et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res. 2015;63:28-35.
3. Aslan S, Lu H. On the sensitivity of asl mri in detecting regional differences in cerebral blood flow. Magn Reson Imaging. 2010;28:928-935.
4. Melie-Garcia L, Sanabria-Diaz G, Sanchez-Catasus C. Studying the topological organization of the cerebral blood flow fluctuations in resting state. NeuroImage. 2013;64:173-184.
Figures
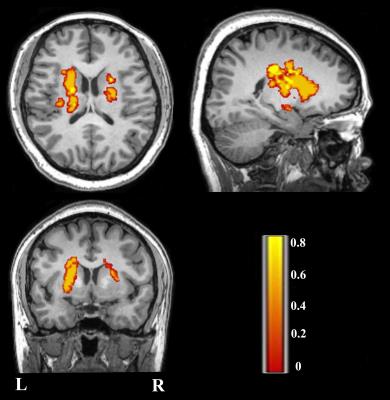
Fig 1: Lesion incidence map of patients with stroke. Color represents lesion incidence frequency.