1710
Towards specific biomarkers of chronic pain: Modelling behavior readouts of developing pain to resting-state functional connectivity using a developmental trajectory in a mouse model of chronic pain from bone cancer1Inst. for Biomedical Engineering, ETH & University of Zurich, Zurich, Switzerland, 2Neuroscience Center Zurich, Zurich, Switzerland, 3Singapore Bioimaging Consortium, Singapore, Singapore, 4Inst. for Pharmacology & Toxicology, University of Zurich, Zurich, Switzerland
Synopsis
We performed longitudinal behavioral readouts of pain and resting-state fMRI in a mouse model of chronic pain from breast cancer derived tibial bone metastases. The developmental trajectory of behavioral readouts was used to model the fMRI response to extract pain-specific functional connectivity changes during development of a chronic pain state. The specificity of these functional readouts was supported through inhibition of osteolytic activity, reducing the nociceptive input. Thereby, characteristic functional changes could be reduced and in some regions prevented. These results emphasize the specificity of the functional readouts for developing chronic pain and could be used to evaluate novel treatments.
Purpose
Chronic pain affects 15% of the world’s population and poses a major burden on patients’ lives due to insufficient treatment options1. Development of novel treatments has been largely unsuccessful due to poor translatability of preclinical findings, typically based on behavioral readouts of pain. They probe simple reflexes or innate responses, which are a result of sub-spinal processing rather than actual pain generated in the brain2. This could be problematic in particular in chronic pain conditions where emotional and affective components were found to be main contributors in the chronification of pain3. We therefore performed longitudinal analysis of functional connectivity based on resting-state fMRI to find potential signatures of developing chronic pain in the brain, which might serve for evaluating novel treatments. Specificity of these readouts was tested by modulating the nociceptive input using peripherally active treatments.Material & Methods
Mouse
models of chronic pain from bone cancer were prepared by intramedullar injection
of EO771 (tebu-bio) breast cancer cells into the tibia of female C57BL/6 (n=10).
Functional changes in the brain were assessed longitudinally using a Bruker
Biospec 94/30 small animal MR system with a four-element receive only cryogenic phased
array coil (Bruker BioSpin AG) with a linearly polarized room temperature
volume resonator for transmission. Resting-state functional MRI (rs-fMRI) data
was acquired using a gradient-echo echo-planar imaging (GE-EPI) sequence:
TR=1000ms, TE=12ms, FA=60°. Ex-vivo digital
radiographs were acquired (35kW/10s) using a planar x-ray system (MX20,
Faxitron). Animals were anesthetized using a combination of medetomidine-hydrochloride
(0.05mg/kg as bolus, 0.1mg/kg/h maintenance, s.c.), intubated and ventilated
with low dose isoflurane (0.5%) in 20%O2 / 80%air mixture at
80breaths/min. For immobilization, pancuronium bromide was administered s.c. in
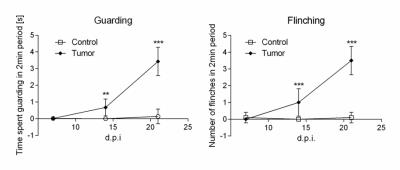
a bolus of 0.5mg/kg. Behavioral readouts of pain were assessed in terms of time
spent guarding and number of flinches in a 2min period. Rs-fMRI data was
analyzed using independent component analysis (ICA). Longitudinal evolution of
obtained components was modelled on a developmental trajectory obtained from
behavioral readouts of pain. Statistical differences between groups were then
tested using a general linear model with non-parametric permutation testing,
accounting for multiple comparisons with threshold-free cluster enhancement and
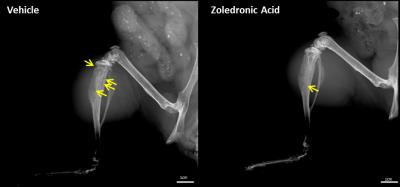
Bonferroni correction. Treatment with zoledronic acid (Zoledronate Onco Sandoz,
Sandoz) was administered prospectively 7days post tumor injection (10mg/kg,
s.c.).Results
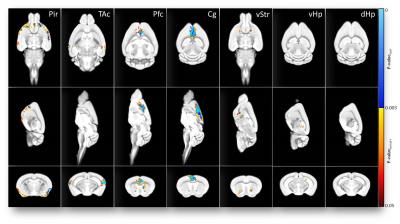
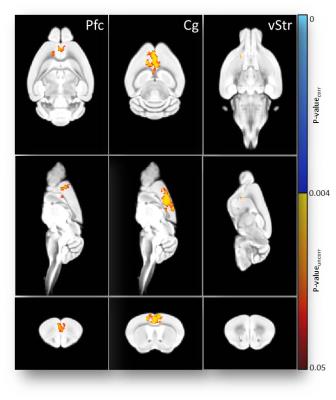
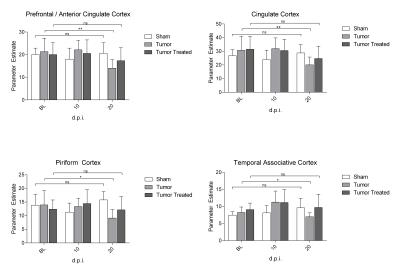
Nociceptive behavior was significantly increased 14 and 21days post injection in tumor-bearing compared to sham treated animals (Fig.1). Both guarding and flinching indicated similar longitudinal evolution of nociceptive behavior, supporting the trajectory used for modelling the fMRI response. Most robust functional changes were found in the cingulate (p=0.0004) and prefrontal/anterior cingulate cortex (p=0.0006). Additionally, piriform (p=0.0008) and temporal associative cortices (p=0.0002) were found to be affected as well as the ventral striatum (p=0.003). Less robust changes were observed in the ventral (p=0.009) and dorsal (p=0.033) hippocampus (Fig.2). Prospective treatment with zoledronic acid has been shown to effectively reduce osteolysis4 and efficacy could be confirmed in ex-vivo digital radiographs (Fig.3). Tibiae of treated mice indicated only minor signs of osteolysis, which were highly abundant in vehicle-treated animals. Reduced peripheral input significantly modulated the central readouts described before (Fig.4). Within-network connectivity in the cingulate (p=0.0053) and prefrontal/anterior cingulate (p=0.012) cortex and ventral striatum (p=0.0022) was found significantly reduced but still remained above statistical significance although it did not survive Bonferroni correction. Changes in the piriform (p=0.39), temporal associative (p=0.24), dorsal (p=0.15) and ventral (p=0.071) hippocampus did not reach significance after treatment (Fig.5).Discussion
Longitudinal rs-fMRI data was modelled on a trajectory obtained from behavioral readouts of pain to find regions specific to developing chronic pain. Several regions were found to be affected, following the inverse developmental trajectory of behavioral readouts. The observed functional alterations in brain connectivity were found highly robust and reproducible with a maximum effect size of 30%. These functional changes could be modulated by peripherally active therapy, supporting the specificity of the presented readouts. Complete inhibition of central effects was not achieved, since only one aspect of pain was targeted in the applied treatment regime. The maximum effect-size of the treatment in the prefrontal/anterior cingulate cortex of 20% is comparable to clinical data of patients suffering from metastatic bone cancer treated with zoledronic acid5.Conclusion
By using behavioral readouts of pain
we found direct functional correlates of developing chronic pain in the brain.
These readouts are modular through application of treatments, therefore seem to
be specific. Given the reproducibility and effect-size, these readouts could be
used to evaluate novel treatments. Since pain is ultimately generated in the
brain, these central readouts might be much more specific than nocifensive
behavioral readouts, which potentially enhances translatability of preclinical
pain research.Acknowledgements
This study was supported by the Swiss National Science Foundation (grant SNF 160310, MR).References
1. Murray CJL, Lopez AD. Measuring the Global Burden of Disease. N Engl J Med. 2013;369(5):448-457. doi:10.1056/NEJMra1201534.
2. Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283-294. doi:10.1038/nrn2606.
3. Baliki MN, Apkarian A V. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87(3):474-491. doi:10.1016/j.neuron.2015.06.005.
4. Casanova M, Herelle J, Thomas M, et al. Effect of combined treatment with zoledronic acid and parathyroid hormone on mouse bone callus structure and composition. Bone. 2016;92:70-78. doi:10.1016/j.bone.2016.08.012.
5. Saad F, Gleason DM, Murray R, et al. Long-Term Efficacy of Zoledronic Acid for the Prevention of Skeletal Complications in Patients With Metastatic Hormone-Refractory Prostate Cancer. JNCI J Natl Cancer Inst. 2004;96(11):879-882. doi:10.1093/jnci/djh141.
Figures