1709
Disruption of Functional Connectivity in Neonates with Hypoxic Ischemic Encephalopathy at Term1Center for Metabolic Imaging & Therapeutics, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland Baltimore School of Medicine, Baltimore, MD, United States, 2Pediatrics, University of Maryland Baltimore School of Medicine, Baltimore, MD, United States
Synopsis
Perinatal hypoxic-ischemic encephalopathy (HIE) is one of the most common causes of severe, long-term neurologic deficits in children. Up to 40% of infants with HIE who present minimal to no abnormality on structure MRI may still have manifest neurological deficits in later life. In this study, we hypothesize that functional connectivity (FC) of the brain in infants with HIE at term may be altered and that resting-state functional MRI (rs-fMRI) may provide deeper insights into the altered nature of the brain networks, especially the motor network and the thalamocortical network in infants with HIE.
Purpose
Purpose: Perinatal hypoxic ischemic encephalopathy (HIE) occurs in 1-6 of 1000 term births and is one of the most common causes of severe, long-term neurologic deficits in children1. Structural abnormalities in the basal ganglia and thalami noted on magnetic resonance imaging (MRI) are highly predictive of major neuromotor and cognitive deficits in neonates with HIE. However, up to 40% of infants with HIE who present minimal to no abnormality on conventional structure MRI may still have manifest neurological deficits in later life 2. In this study, we hypothesize that functional connectivity (FC) of the brain in infants with HIE at term may be altered and that resting-state functional MRI (rs-fMRI) may provide deeper insights into the altered nature of the brain networks, especially the motor network and the thalamocortical network in infants with HIE.Materials and Methods
Sixteen term infants with HIE (14.2±12.5 days, 11M/5F) and nine age-matched controls (14.6±13.5 days, 6M/3F) received MRI scans using a 3T Tim-Trio Siemens system during natural sleep at University of Maryland Medical Center. High-resolution T1-MPRAGE images were acquired with parameters (TR/TE 1900/3.8ms; flip angle 15o, voxel size of 1x1x1 mm3). Based on the clinical and laboratory criteria, the HIE group demonstrated minimal to no abnormalities on structural MRI determined by a neuroradiologist. The rs-fMRI data was acquired using a T2* weighted EPI sequence with parameters (TR/TE 1720/30 ms, flip angle 80o, voxel size 2.8x2.8x4 mm3 for total 250 volumes). Standard preprocessing for rs-fMRI data were conducted using AFNI (https://afni.nimh.nih.gov/afni/) including time slicing, realignment, co-registration to structural T1 image, normalization to the neonate template provided by the North Carolina University (http://www.nitrc.org/projects/pediatricatlas), spatial smoothing with full-width half- maximum of 6 mm Gaussian kernel, temporal band filtering between 0.008 and 0.09 Hz. Six motion parameters and their first derivatives and the average global signal of the brain mask were regressed out as nuisances. Spherical ROIs of 4mm were placed at the bilateral primary motor cortex (M1), supplemental motor area (SMA), premotor area (PMA), and thalamus. General linear model was utilized to estimate the FC map between the M1 and thalamus and the rest of the brain. The Fisher-z transformation was performed on the correlation coefficient map for normalization. One sample t-test with family wise error (FWE) correction p < 0.05 was used for statistic FC map within controls and HIE group. Two sample t-test with voxelwise uncorrected p < 0.002 and excluded voxel size of 50 was used for statistic FC map between control and HIE groups. We also calculated Pearson correlation matrix for paired ROIs within the motor network and its Fisher-Z FC matrix. A mean FC index in the motor network was also calculated for the two groups separately.Results
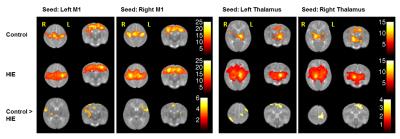
The results of within group FC maps in Figure 1 show that robust and strong inter- and intrahemispheric functional connectivity is present within the motor and thalamocortical networks. The group difference FC maps in the left panel show significant reduction of inter- hemispheric FC between the M1 and other components within the motor network and auditory cortex. The calculated mean motor FC indices (control: 0.529 ± 0.18, HIE: 0.405 ± 0.17, two sample t-test p < 0.04) also show a significantly decreased FC within the motor network in HIE group. In the thalamocortical network (right panel), the infants with HIE demonstrate reduced FC between the left thalamus and the ipsilateral motor cortex and contralateral visual cortex, as well as between the right thalamus and its contralateral motor cortex, somatosensory cortex and prefrontal cortex.Discussion and Conclusion
Disruption of functional connectivity within the motor and the thalamocortical networks may be presented in infants with HIE despite the absence of significant abnormalities in structural images. Such disruption of functional connectivity may underlie the motor, visual, hearing and cognitive deficits experienced by these infants in their later life. Therefore, the early characterization of such deficits measured by rs-fMRI may hold the potential to predict the neurodevelopmental outcome.Acknowledgements
No acknowledgement found.References
1. Perez, A., Ritter, S., et al (2013). Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. The Journal of pediatrics, 163(2): 454-459.
2. Rollins, N., Booth, T., et al (2014). Predictive value of neonatal MRI showing no or minor degrees of brain injury after hypothermia. Pediatric neurology, 50(5): 447-451.