1703
Patterns of the whole brain default mode network for alcohol addiction among the alcohol preferring rats1Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan, People's Republic of China, 2Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, Wuan, People's Republic of China, 3Department of Anesthesiology, Peking University Third Hospital, Beijing, People's Republic of China, 4Insititute of High Energy Physics, Chinese Academy of Sciences, Beijing, People's Republic of China
Synopsis
Alcohol preferring and non-preferring rats were trained with two-bottle choice methods. The whole brain default mode network was acquired during different period of addiction training and after alcohol injection. The correlation coefficients among 28 regions were calculated by mean-value of Pearson correlation. The changes of brain connections after alcohol consumption in different stage of alcohol addiction is significant different. Furthermore, some addiction or reward associated regions and new possible areas (NAc, Tu, VTA, IC, Hypo, SNC, Au, DB, Hipp, VLPO, etc.) showed difference during the training. Our findings could provide preliminary functional connection support of neurobiological circuits change.
PURPOSE
Alcohol addiction, regarded as a chronically relapsing disorder, affects millions of people during their lives especially on the impairments of emotion and cognitive through a whole brain neurocircuitry.The studies of appropriate animal models, based on global network functional magnetic resonance imaging(fMRI) method for calculating the default network alterations during different addiction stages, can be effect on better understanding of the neurobiology of addiction from the point of multiple. 1METHODS
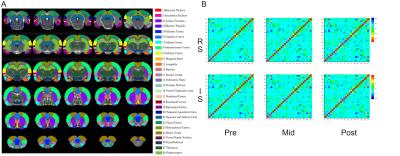
Two animal models(Alcohol preferring and non-preferring rats, P-rat and NP-rat) 2 have been trained for three different stages(Pre-0, Mid-2weeks, Post-4weeks) of alcohol addiction with two-bottle choice method (5% alcohol). To examine changes in whole brain neural response to alcohol in the development of alcohol addiction, 11 P-rats, 9 NP rats(19-60 trials per state, 195 trials in total) were scanned by 7.0T small animal scanner with resting state functional magnetic resonance imaging(fMRI) methods(EPI-FID) both before and after 0.76g/kg alcohol injection(ip). The correlation coefficients among28 regions were calculated by mean-value of Pearson correlation 3. Some addiction related regions(NAc/Tu/DB/VTA) were also calculated through a pixel-by-pixel analyzing.RESULTS
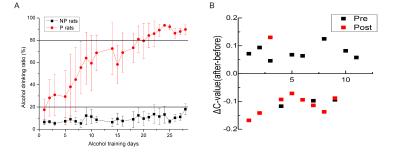
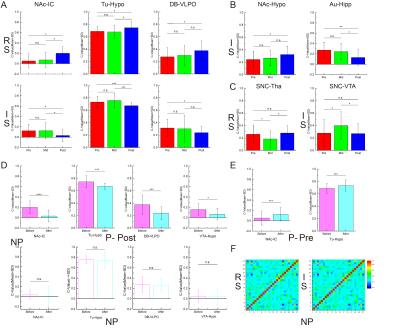
The daily intakes of alcohol by self-selection for P- and NP-rats are illustrated in Fig.1A. The alcohol drinking ration for the P rats increases with the training days and finally reached up to >80%. And for NP rats, the ratio is <20% throughout the whole training. For rest state analysis, the changes of brain connections after alcohol consumption in different stage of alcohol addiction is significant different. Most of the significant changes of brain region connections(8/9) were decreased in post-period while the significant changes of paired regions(8/11) are increased in pre-period(Fig.1B) by alcohol stimulation. For 28 selected brain regions in the whole brain (Fig. 2A), the mean correlation coefficient matrices(Fig.2B) of most regions after alcohol injection do not change during three different stages. However, some addiction or reward associated regions and new possible areas(NAc, Tu, VTA, IC, Hypo, SNC, Au, DB, Hipp, VLPO, etc.) showed difference during the training. Among these changes, three pairs(NAc-IC, Tu-Hypo, DB-VLPO) emerged significant increments(p<0.001~0.05) between Pre- and Mid-/Post- stages before alcohol injection(restingstate, RS), whereas, decrements after alcohol injection(intoxicated state, IS)(Fig.3A).The coherence of NAc-Hypo(IS)in Post- state got a significant increment(p<0.05) comparing with Pre- state, while the coherence of Au-Hipp(IS) got a reduction from Pre-/Mid- to Post-(p<0.01~0.05)(Fig.3B). Notably, the significant differences of the correlation coefficients for SNC-Tha(RS) and SNC-VTA(IS) are only observed in Mid-period(p<0.05) (Fig.3C). Furthermore, the upper changes of relevant areas did not appeared in NP rats at all during different stages (Fig.3D).DISCUSSION
The variety of coherence pattern is considered to relate with the development of alcohol addiction. The results of the whole connections of brain regions showed that it is a dynamic change of multiple neurobiological circuits during a long term alcohol addiction. These areas(NAc, Tu, VTA, IC, Hypo, SNC, Au, DB, Hipp, VLPO) mainly involve reward, motivation, emotion and cognitive circuits which could independently or interactively lead to corresponded addictive behaviors 4. Furthermore, there is no directed structural inputs or outputs connection in some intensive functional connection(BOLD-signal-dependence) regions(NAc-IC, etc) which indicates some complex factors involved(like mini-circuits, neurotransmitter, multiple neurotransmitter-specific neuroplasticity circuits). Moreover, the changes only happen during the mid-term(SNC-Tha and SNC-VTA) relevant to dopaminergic system suggested that this part of dopamine pathway may only act during the period of addiction develops rather than maintains. Since fMRI only roughly estimate the connection among different regions, it is important to further analyze the changes of the founding with a more precise region differentiating template at the network level. Furthermore, the identification of molecular and neurochemical related with the changes of these circuitries, as well as genetic factors occasions should be the further prospects to solve the beset of alcohol disorder.CONCLUSION
Our investigation was major focus on the changes of the whole brain connections of alcohol specify drinking rat models during the development of alcohol addiction period. Our findings could provide preliminary functional connection support of neurobiological circuits change, highlighting the critical importance of stage-different networks.Acknowledgements
The work was supported by Chinese Ministry of Science and Technology (2015AA020508).References
1. Koob G, Volkow N, Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016. 3(8): 760-773.
2. Lumeng L, Hawkins T, Li T, New strains of rats with alcohol preference and nonpreference. Alcohol and aldehyde metabolizing systems. 1977. 3:537-544.
3. Nie B, Chen K, Zhao S, A rat brain MRI template with digital stereotaxic atlas of fine anatomical delineations in paxinos space and its automated application in voxel-wise analysis. Hum Brain Mapp. 2013. 34(6): 1306-18.
4. Koob G, Volkow N, Neurocircuitry of addiction. Neuropsychopharmacology. 2010. 35(1): 217-38.
Figures