1702
Disrupted Cortical Modulation of Hypothalamus Functioning for Visceral Homeostasis in Patients with Irritable Bowel Syndrome1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Gastroenterology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Pediatric Gastroenterology, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
The hypothalamus plays a critical role in maintaining visceral homeostasis. We evaluated, using functional imaging, hypothalamus functional connectivity in adolescent IBS patients and controls who received rectal distension stimulations. More extensive hypothalamus connectivity was observed in liminal than subliminal condition in controls, but not in IBS patients. Compared with controls, IBS patients showed significantly reduced hypothalamus connectivity in the bilateral prefrontal cortices, supplementary motor and premotor areas, bilateral sensorimotor cortex, and limbic subareas, which are specifically involved in homeostatic regulation. The findings support that reduced cortical and limbic modulations of hypothalamus functioning underlies disrupted visceral homeostasis in IBS patients.
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (FGID) disorder characterized by chronic abdominal pain or discomfort with altered bowel habits.1 IBS symptoms can be characterized by dysfunctions in a generalized model of visceral homeostatic regulation network within the brain-gut axis.2 The hypothalamus plays a critical role in regulating visceral homeostasis through its intimate connections with the autonomic nervous system, neuroendocrine system, and limbic system.3 One important aspect of maintaining visceral homeostasis is the cortical modulation of homeostatic afferents. 2 Prefrontal, limbic, paralimbic, and sensory and motor cortices all can exert modulatory influences on the gains of autonomic reflexes. A set of prefrontal regions modulates activities in limbic and paralimbic regions, subregions of the anterior cingulate cortex (ACC), and the hypothalamus, which in turn regulate activities of descending inhibitory and facilitatory pathways through the periaqueductal gray (PAG) and pontomedullary nuclei.2 The interactions in these corticolimbic pontine networks are thought to mediate the cognitive and emotional aspects of homeostatic afferents, including visceral pain and discomfort. The goal of this study was to evaluate, using functional imaging, alterations of hypothalamus functional connectivity in adolescent IBS patients. We hypothesized that IBS patients are characterized by reduced hypothalamus functional connectivity with cortical and limbic structures that are specifically involved in homeostatic regulation, supporting a lack of cortical modulation on hypothalamus functioning as a mechanism of disrupted visceral homeostasis in IBS patients.Methods
Study participants included nine adolescent IBS patients aged 12–17 years and eight age-matched healthy volunteers (controls). All patients meet Rome III criteria for the diagnosis of IBS. Two scans were performed during each of the subliminal and liminal non-painful rectal balloon distension stimulations administered using a commercially available computer-controlled barostat. The average balloon pressures that elicited subliminal and liminal stimulation were at 15±4 mmHg and 24±4 mmHg for IBS patients, and 16±6 mmHg and 26±5 mmHg for controls, respectively. No participant reported feeling of pain during liminal stimulation. Each fMRI run consisted of four repeating cycles, with each cycle comprising a 15-second pressure and a 25-second rest. The hypothalamus was manually identified in high spatial-resolution anatomical images of each participant as the seed region for a functional connectivity analysis.Results
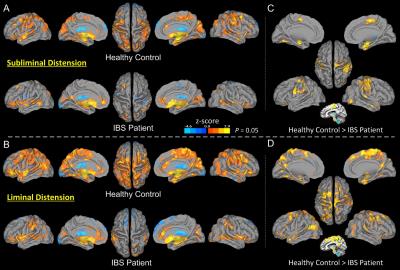
The control and IBS groups showed different spatial patterns of hypothalamus functional connectivity in the subliminal and liminal rectal distension conditions (Fig. 1A, 1B). Controls showed extensive connectivity with multiple cortical areas while IBS patients did not. Common hypothalamus connections were found in both groups in the ACC (more in the pregenual ACC [pACC] in IBS patients and dorsal ACC [dACC] in controls), bilateral insula, middle and superior temporal gyrus, amygdala, thalamus, hippocampus, parahippocampal cortex, uncus; basal ganglion structures including the putamen, globus pallidus and caudate; and cerebellum subregions. Controls, not IBS patients, however, showed additional widespread connectivity with a large set of cortical regions in the bilateral inferior and middle frontal gyrus, dACC, bilateral sensorimotor areas, supplemental motor area, premotor cortex, inferior and superior posterior parietal lobe, angular and supramarginal gyrus, cuneus, and part of the middle cingulate cortex. In both groups, prominent hypothalamus connections with the midbrain structures, PAG, parabrachial nucleus, and other subareas of the pons were observed. Compared with subliminal stimulation, there was a prominent increase in the extent and magnitude of hypothalamus connectivity in all involved cortical regions during liminal stimulation in controls, but not in IBS patients. Group comparisons revealed a trend of between-group differences similar to those observed in individual group maps in the subliminal (Fig. 1C) and liminal (Fig. 1D) distension conditions.Discussion & Conclusion
Our study identified that hypothalamus functional connectivity in adolescent IBS patients was reduced in neural structures specifically involved in sensory-discriminative, cognitive-evaluative, and affective-motivational aspects of the CNS processing of visceral sensation and pain.1 Degraded hypothalamus communication along the descending pathway to brainstem nuclei was also implicated. Our study supports that the disrupted cortical modulation of hypothalamus functioning is an important contributing factor, in the context of the generalized homeostatic regulation network2, to the disruption of visceral homeostasis in adolescent IBS patients.Acknowledgements
Research presented in this publication was supported in part by a grant from the Digestive Diseases Center, Medical College of Wisconsin, and National Institutes of Health (NIH) grants R01GM103894 and R01DK025731. We thank Lydia Washechek, BA, for editorial assistance.References
1. Aziz Q and Thompson DG. Brain-gut axis in health and disease. Gastroenterology, 1998; 114(3):559-78.
2. Mayer EA, Naliboff BD, and Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology 2006; 131(6):1925-42.
3. Saper CB and Lowell BB. The hypothalamus. Curr Biol, 2014; 24(23):R1111-6.