1701
Higher Thalamocortical Connectivity May Reduce Efficacy of Temporal Resection1Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 2Psychiatry, Vanderbilt University Medical Center, Nashville, TN, United States, 3Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN, United States, 4Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 5Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
Functional connectivity of the thalamus could inform the potential efficacy of temporal resection in temporal lobe epilepsy. Diffusion and functional MRI was collected from 22 patients who underwent surgical treatment for temporal lobe epilepsy. The thalamus was segmented into six subregions based on diffusion tractography to six regions encompassing the entire cortex. Functional connectivity was calculated between each thalamic subregion and cortical region for each patient. Higher functional connectivity between the contralateral temporal-thalamic subregion and the cortex was associated with seizure recurrence after surgery.
Purpose
Epilepsy affects 1% of the population and 30% of these patients have drug-resistant seizures1. In temporal lobe epilepsy (TLE), seizures originate in the temporal lobe, but the epileptogenic network can include several extra-temporal regions2. While temporal resection is the most effective treatment for drug-resistant TLE, seizure freedom is not attained in 20-50% of patients3,4. There may be subtypes of TLE, which could be defined by connectivity in the epileptogenic network external to the temporal lobe5, and different treatments may have distinct efficacies in different TLE subtypes. The thalamus is a central node in the TLE epileptogenic network: important for the spread of seizures to cortical regions6 and an effective site for electrical stimulation for seizure reduction7. Analysis of brain connectivity has shown some clinical promise for lateralizing TLE and predicting surgical outcome8-10. The purpose of this work is to determine if thalamocortical functional connectivity is associated with seizure recurrence after temporal resection. Characterizing thalamic functional connectivity could deepen our understanding of network effects in TLE and begin to identify which connectivity changes may predict treatment response in individual patients.Methods
Diffusion and resting-state functional MRI was collected from 22 unilateral TLE patients who subsequently underwent temporal resection. Using diffusion tractography, the thalamus was divided into subregions based on structural connectivity between each thalamic voxel and six cortical regions (Figure 1), for both ipsilateral and contralateral hemispheres relative to seizure focus11. Functional connectivity (FC) was then calculated between each thalamic subregion and six cortical regions. To explore if thalamic connectivity affects outcome after temporal resection, we compared patients who continued to have disabling seizures (Engel 2-4) vs. those who were seizure-free (Engel 1) one year after surgery12. To compare these patient groups, t-tests were performed for all connections between each thalamic subregion and cortical region, with further analyses using average FC across cortical regions for each temporal subregion.Results
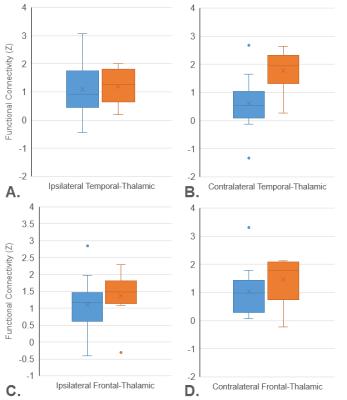
Across 36 thalamocortical connections in each hemisphere there were no individual connections with a difference between the groups with p<0.01. In analyzing the average cortical connectivity to the six thalamic subregions the contralateral temporal-thalamic subregion showed the largest group difference (Figure 1B, p=0.0061) with higher connectivity in Engel 2+ patients. Figure 1A presents average cortical connectivity from the ipsilateral temporal-thalamic subregion showing a much smaller group difference in the thalamic subregion directly connected to the epileptogenic lobe (p=0.1175). For comparison from another temporal subregion, Figures 1C and 1D present ipsilateral and contralateral frontal-thalamic subregions also showing minimal group differences (p=0.3810 and p=0.2917).Discussion
Previous research demonstrated that brain connectivity may be related to postsurgical outcome9. Our data suggest that higher FC between the contralateral temporal-thalamic subregion and cortex is associated with seizure recurrence after temporal lobectomy. The ipsilateral temporal-thalamic subregion and other thalamic subregions did not demonstrate a clear difference between patient groups. This data could indicate that some of the contralateral thalamus may have been recruited into the epileptogenic network before surgery, restarting the epileptogenic network after surgery. Further research should investigate whether thalamic connectivity patterns can predict seizure recurrence after temporal resection with a different and larger TLE cohort. Thalamocortical connectivity may also influence the efficacy of other seizure reduction treatments, especially electrical stimulation of the thalamus7. This work presents the first steps identifying thalamocortical connectivity as a diagnostic measure for the treatment of epilepsy.Acknowledgements
NIH R01 NS075270 (VLM)References
1. Helmstaedter, C. & Kockelmann, E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia 47 Suppl 2, 96-98, doi:10.1111/j.1528-1167.2006.00702.x (2006).
2. Spencer, S. S. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 43, 219-227 (2002).
3. Spencer, S. & Huh, L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 7, 525-537, doi:10.1016/S1474-4422(08)70109-1 (2008).
4. Englot, D. J. et al. Seizure types and frequency in patients who "fail" temporal lobectomy for intractable epilepsy. Neurosurgery 73, 838-844; quiz 844, doi:10.1227/NEU.0000000000000120 (2013).
5. Bonilha, L., Martz, G. U., Glazier, S. S. & Edwards, J. C. Subtypes of medial temporal lobe epilepsy: influence on temporal lobectomy outcomes? Epilepsia 53, 1-6, doi:10.1111/j.1528-1167.2011.03298.x (2012).
6. Rosenberg, D. S. et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia 47, 98-107, doi:10.1111/j.1528-1167.2006.00375.x (2006).
7. Fisher, R. et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51, 899-908, doi:10.1111/j.1528-1167.2010.02536.x (2010).
8. Bettus, G. et al. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. Journal of neurology, neurosurgery, and psychiatry 81, 1147-1154, doi:10.1136/jnnp.2009.191460 (2010).
9. Bonilha, L. et al. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 81, 1704-1710, doi:10.1212/01.wnl.0000435306.95271.5f (2013).
10. Morgan, V. L., Sonmezturk, H. H., Gore, J. C. & Abou-Khalil, B. Lateralization of temporal lobe epilepsy using resting functional magnetic resonance imaging connectivity of hippocampal networks. Epilepsia 53, 1628-1635, doi:10.1111/j.1528-1167.2012.03590.x (2012).
11. Behrens, T. E. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature neuroscience 6, 750-757, doi:10.1038/nn1075 (2003).
12. Engel, J. Surgical treatment of the epilepsies. 2nd edn, (Raven Press, 1993).
Figures