1697
Greater Connectivity Between Cerebellar Vermis and Insular Attention Resting Network in HIV Patients1John A Burns School of Medicine, University of Hawaii at Manoa, Honolulu, HI, United States
Synopsis
Individuals infected with HIV frequently have HIV-associated neurocognitive disorders (HAND), despite effective plasma viral suppression. Various resting state networks studied with fMRI have been shown to be attenuated in HIV compared to seronegative controls. This study shows an increased connectivity in HIV subjects between the cerebellar vermis and bilateral insulae. Using clinical correlates, we conclude that this increased connectivity may be due to decreased efficiency in functional connectivity in the HIV-infected brain.
INTRODUCTION
Individuals infected with HIV frequently have HIV-associated neurocognitive disorders (HAND),1 despite effective plasma viral suppression. Resting state networks (RSNs) studied with fMRI also have been shown to be attenuated in those with HIV compared to seronegative controls.2-3 The cognitive dysfunction and abnormal brain activities in these patients are hypothesized to be related to the persistence of viral reservoirs in the brain, which leads to ongoing neuro-inflammation.4 The aim of the current study is to further characterize HIV-associated alterations in connectivity of specific canonical RSNs and to correlate these effects to clinical variables, including the performance on neuropsychological tests.METHODS
134 participants [70 seronegative (SN) subjects: ages 51.6 ± 1.4 years, 64(91%) men; 64 HIV-infected subjects: ages 50.8 ± 1.3 years, 59(92%) men] were assessed with a detailed structured neuropsychiatric evaluation, including HIV disease severity for the HIV+ patients and substance use histories to ensure they fulfilled study criteria. All subjects were scanned using a 3 Tesla MRI scanner (Tim Trio, Siemens Medical Solutions, Erlangen Germany) with a 12-channel phase-array RF head coil. Two echo planar imaging sequences with TR=3000ms TE=30ms, FOV=120, 42 slices with 3mm isotropic voxels were acquired while the subject was instructed to lie in the scanner with eyes closed and remain motionless. A structural imaging sequence was also performed (Magnetization Prepared Rapid Gradient Echo, TR=2200ms, TE=4.48ms, TI=1000ms with 1mm isotropic voxels) for subject level co-registration during analysis. Functional connectivity was evaluated using the Conn toolbox (www.NITRC.org). All imaging data were de-noised at the subject level with use of structural and segmentation maps prior to performing the toolbox’s independent component analysis of the functional data. The results were corrected for multiple comparisons using the False Discovery Rate (FDR) method. Age was added as a covariate in all analyses. Clinical assessments: Each participant was Each participant was also administered a battery of neuropsychological tests that included 7 cognitive domains (Fluency, executive function, speed of information processing, attention/working memory, learning, memory and motor skill) in order to determine if they had HAND.RESULTS
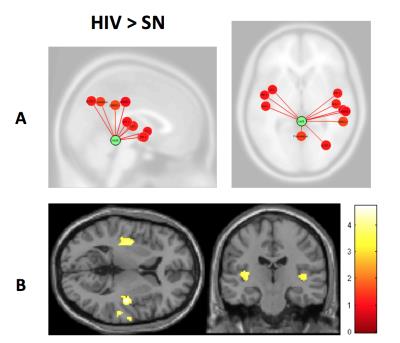
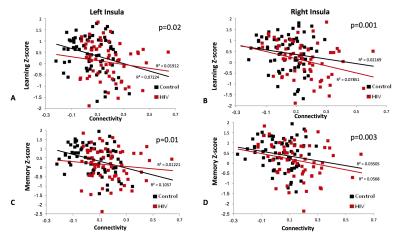
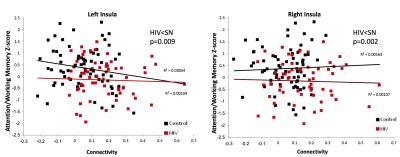
Participant characteristics: Both groups were majority white males (59% in SN; 64% in HIV). CES-Depression score was non-significantly higher in HIV than SN, but both groups were not clinically depressed. The SN group also had higher Karnofsky score compared to HIV subjects (p<0.001). HIV-subjects performed significantly worse on 4 of the 7 cognitive domains: speed of information processing, attention/working memory, learning and memory (Table 1). Imaging: Compared to SN controls, HIV-subjects had greater connectivity between the cerebellar vermis and bilateral insulae (Figure 1). The BOLD signal within connectivity seeds showed an inverse correlation between insula-vermis connectivity and performance in the learning (Figure 2A-B) and memory domains (Figure 2C-D). A HIV main effect was also shown between bilateral insulae and the attention/working memory domain (Figure 3). Lastly, within the HIV group, compared to those without HAND (n=49), HIV subjects with HAND (n=15) had lesser connectivity between the left hippocampus and the ipsilateral operculum (FDR-p=0.039).DISCUSSION
Contrary to prior studies that showed decreased connectivity in various canonical RSNs in HIV compared to SN controls,2,5-7 the current study shows increased connectivity in an RSN within the attention network in HIV patients. We further showed that those with greater connectivity had poorer performance on learning and memory. These findings suggest that the participants with poorer cognitive performance had lesser network efficiency and had to compensate by increasing their functional connectivity within this network. Our findings are consistent with prior reports that showed greater brain activation in HIV subjects with HAND or poorer cognitive performance.8 The lesser network efficiency may in part be due to decreased dopaminergic function.9-10 The cerebellar vermis has been shown to be involved in cognitive function through its dopaminergic-mediated pathways.11 Therefore, our findings of lesser efficient cerebellar-insular RSN in HIV patients and the associated poorer learning and memory provide further support to the notion that dopaminergic deficit plays a key role in HAND. Lastly, the decreased connectivity between the operculum and the hippocampus in HIV-HAND subjects provides further evidence that deficits in the memory networks may contribute to memory impairment reported in HIV patients.12Acknowledgements
The authors thank the research participants who enrolled in this study, all of our clinical and technical research staff who helped with the data collection, and the community HIV care providers (especially Drs Drew Kovach, Cyril Goshima, and Jennifer Frank), who referred many of their patients to our study. We thank Dr Eric Miller for his valuable advice on the cognitive testing. This workwas supported by the National Institute on Mental Health (2R01-MH061427), the National Institute on Drug Abuse (K24-DA016170; K02-DA016991), the National Institute for Research Resources (G12-MD003061 and U54-NS056883), and the Office of National Drug Control Policy.References
1. Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol. 201 Apr;12(4):234-48
2. Ipser JC, Brown GG, Bischoff-Grethe A, Connolly CG, Ellis RJ, Heaton RK, Grant I; Translational Methamphetamine AIDS Research Center (TMARC) Group.. HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. J Int Neuropsychol Soc. 2015 Mar;21(3):203-13.
3. Ann HW, Jun S, Shin NY, Han S, Ahn JY, Ahn MY, Jeon YD, Jung IY, Kim MH, Jeong WY, Ku NS, Kim JM, Smith DM, Choi JY. Characteristics of Resting-State Functional Connectivity in HIV-Associated Neurocognitive Disorder. PLoS One. 2016 Apr22;11(4):e0153493.
4. Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, Ernst T. Effects of APOE ε4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014 Jun 17;82(24):2213-22.
5. Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013 Mar 26;80(13):1186-93.
6. Wang X, Foryt P, Ochs R, Chung JH, Wu Y, Parrish T, Ragin AB. Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain Connect. 2011;1(3):207-17. 7. Ances BM, Christensen JJ, Teshome M, Taylor J, Xiong C, Aldea P, Fagan AM, Holtzman DM, Morris JC, Mintun MA, Clifford DB. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case-control study. Neurology. 2010 Jul 13;75(2):111-5.
8. Chang L, Holt JL, Yakupov R, Jiang CS, Ernst T. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging. 2013 Apr;34(4):1240-53.
9. Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004 Nov;127(Pt 11):2452-8.
10. Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008 Aug 15;42(2):869-78.
11. Anderson CM, Maas LC, Frederick Bd, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006 Jun;31(6):1318-26.
12. Wang M, Wang Q, Ding H, Shang H. Association of Hippocampal Magnetic Resonance Imaging With Learning and Memory Deficits in HIV-1-Seropositive Patients. J Acquir Immune Defic Syndr. 2015 Dec 1;70(4):436-43.
Figures
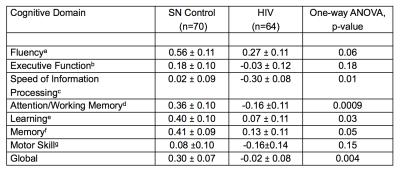
Table 1: Cognitive Performance (Cognitive Domain Z-Scores, Mean±S.E.)*
*Z-scores are derived from a normative database of 481 seronegative healthy controls, and are adjusted for age and education
a RFFT and Verbal Fluency
b Stroop Interference and Trail Making Test B
c Symbol Digit, Trail Making Test A, Stroop Color Naming, and California Computerized Assessment Package Simple Reaction Time
d WAIS-III Digit Span Backward, Letter-Number Sequencing, Arithmetic and Paced Auditory Serial Addition Test 1
e Rey Auditory Verbal Learning Test Trial 5
f Rey Auditory Verbal Learning Test Delayed Recall
g Grooved Pegboard Dominant and Non-dominant hands.