1696
Low-dose radiation disrupts functional brain connectivity during nociceptive heat stimulation in a mouse model1Institute for Pharmacology and Toxicology, Friedrich-Alexander Universität Erlangen-Nuremburg, Erlangen, Germany, 2Department for Radiology, University Hospital der FAU Erlangen-Nuremberg, Erlangen, Germany
Synopsis
Due to improved medical techniques such as CT and radiotherapy todays children are more exposed to low-dose radiation than previous generations. However, little is known about the influence of low-dose radiation on the brain’s development. We hypothesize that altered functional connectivity of activated areas during pain processing can serve as a marker for disturbed brain development. In a mouse model we showed that functional connectivity during nociceptive stimulation after P10 irradiation is disrupted in cortical areas that mature postnatal. We could demonstrate that pain processing is influenced by low-dose irradiation which is in concordance with the brain’s developmental state.
Introduction
The processing of pain is a complex process, which also involves higher associative regions of the brain1. Thus, disturbances in the development of the brain could affect the central nervous processing of pain. Recent studies suggest that the neuronal development of the brain is sensitive to radiation, not only during the fetal phase, but also in early infancy2. In this study, we used sophisticated graph theoretical approaches to investigated disorders in pain processing as a marker for a basal developmental disorder of the mouse brain after postnatal low-dose whole body irradiation.Methods
Experimental design: 10 mice were whole body irradiated with 500 mGray 10 days after birth. Each of the adult irradiated animals as well as 10 naïve controls underwent an fMRI-session starting with a resting state (RS) measurement followed by a BOLD sequence and a second RS measurement and completed with an anatomical scan. Animals were anesthetized with 1.2% isoflurane in medical air and their body temperature was maintained at 37°C by warm water circulating via the holding cradle.
Image acquisition: MRI experiments were carried out using with a 4.7 T/40 cm horizontal bore actively shielded magnet BioSpec (BRUKER, Germany). Gradient system (200 mT/m) and whole-body birdcage resonator enabled homogenous excitation. An actively RF-decoupled 2x2 mouse phased array head coil was used to acquire brain images. First resting state scan consisted of 300 brain volumes acquired with a T2*-weighted single-shot gradient echo-based Echo Planar Imaging sequence (GE-EPI) covering 22 axial slices of the brain in 2 seconds (total time 10 minutes, TE=25.03 ms., TR= 2000 ms, in-plane resolution 391×391 μm, slice thickness 1 mm, matrix 64 ×64, FOV 25x25 mm). BOLD fMRI data were acquired using the same imaging sequence but with a k-space averaging of 2. A set of single thermal stimuli (40°,45°,50° and 55° ± 1°C, plateau for 5 s, ramp 15 s) was applied three times in 3 min 25 sec intervals by a Peltier element on the right hindpaw. Following a second RS scan, 22 corresponding anatomical T2 reference images (RARE, slice thickness 1 mm, field of view 25×25 mm, matrix 256×256, TR = 3000 ms, TEef =11.7 ms, NEX=5) were acquired.
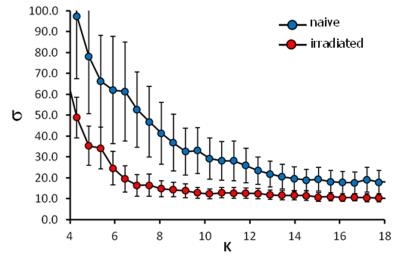
Data processing: After motion correction, GLM analysis with separate predictors for each stimulation temperature, data were FDR thresholded (q<0.05), and different groups of activated voxels were labeled as belonging to 186 pain related brain structures based on the mouse atlas from Paxinos3. For functional connectivity analysis the average time courses of the activated voxels of each brain structure were corrected for global signal fluctuations by linear regression using the global mean as regressor. The graph theoretical approach implements the cross-correlation of residual time courses individually for each stimulus and animal and the resulting 186x186 cross-correlation matrices were averaged over animals. Differences in connectivity strength between groups were calculated using network based statistics (NBS)4. Resting state analysis was performed using a new approach combining classical seed correlation analysis and graph theory. For each animal and brain structure a central seed region was automatically defined and its average time course was correlated with every voxel time course in the brain. After defining the FDR corrected significant correlation voxels an asymmetric correlation matrix was created with the mean significant correlation value per brain structure for each seed region. The efficiency of information flow of the mean resting state networks per group was estimated using the small world index σ5.
Results and Discussion
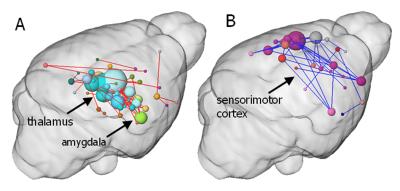
BOLD activation was generally stronger in subcortical brain regions in irradiated than in the control animals. A reduction in activation due to nociceptive stimulation compared to control was observed in the cortex of irradiated animals (Fig. 1). Graph theoretical analysis of the connectivity between areas activated by nociception revealed stronger connections between thalamic regions and disrupted connectivity within cortical areas (Fig. 2). Compared to the control group, the irradiated animals showed a clearly altered RS network before stimulation, which is characterized by disruptive connectivity especially in the area of the "default mode" network. Additionally, the mean RS network of irradiated animals shows a lower small world index compared to the controls (Fig. 3). We conclude that postnatal irradiation with 500 mGray results in an impairment of brain development, which manifests not only in a disruptive RS network with impaired information flow, but also in alterations of the central nervous pain processing. In the latter, especially the higher cortical areas are negatively affected. These structures mature postnatal and therefore are more strongly influenced by radiation.Acknowledgements
BMBF NeuroRad for financial supportReferences
1. Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC neuroscience 2012;13:60.
2. Hall P, Adami H-O, Trichopoulos D, et al. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ 2004;328(7430):19.
3. Franklin KBJ, Paxinos G. The Mouse Brain in stereotaxic coordinates. Academic Press, New York, 3 Ed. 2008
4. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage 2010;53(4):1197-1207.
5. Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature 1998;393(6684):440-442.
Figures