1693
Dependency of the Activations Detected in Resting State Networks on the History of Physical Exercise Activities in Community Dwelling Older Adults1NeuroImaging & Informatics, NCGG, Ohbu, Japan, 2College of Liberal Arts and Sciences, Mie University, Tsu, Japan, 3NeuroImaging & Informatics, National Center for Geriatrics & Gerontology, Ohbu, Japan, 4Faculty of Human Sciences, Kobe Shoin Women's University, Kobe, Japan, 5Department of Radiological Science, Nagoya University Graduate School of Medicine, Nagoya, Japan
Synopsis
In order to explore biomarkers to reflect the effects of long-term physical exercise (PE) on the activities in RSNs, the relationship among the history of participation in PE clubs, the activations in RSNs and the physical activity (PA) was investigated. The activation in the ACG was decreased depending on longer history of PE as well as higher PA indicated by IPAQ scales. These findings may reflect less demand for cognitive integration. It was suggested that both recovery and sedation of the activity in the RSNs against the age-related changes in brain activation may be the biomarker in healthy older adults.
Introduction
It has been known that physical exercise (PE) may potentially improve not only physical health but also cognitive function [1]. Many social programs to support PEs have been conducted as the platforms for health promotion for older adults in many countries. The strategy to delay cognitive impairments is important issues in the aging society, since longer life must be accompanied by continuing opportunities for health, participation and security (active aging). It has been suggested that routine physical activities (PA) in midlife were associated with total brain volume [2] and lower risks of cognitive impairments [3]. Evaluation of resting state networks (RSNs) could be a potential tool for functional characterization of impairment in cognitive functions [4]. Some fMRI studies have shown the relationship between Alzheimer’s disease / mild cognitive impairments and the dysfunction of connectivity in RSNs, especially in the default mode network (DMN) [5]. In this study, we focused on the effects of long-term PE on the activities in RSNs. We investigated the relationship among the period of participation in community based physical exercise clubs (PECs), the activations detected in RSNs and the scores of PAs.Materials & Methods
Seventy-five healthy older adults (Age 61-78, 45 females) recruited from local PECs or an organization for social services, who gave written informed consent approved by the IRB, participated in this study. Their histories of participation in the PECs were interviewed. As behavioral data, the scores of MMSE, GDS, IPAQ (activity and environments) were recorded. Functional images during resting state with eyes open were recorded by using a GRE-EPI sequence (TR 3000ms, TE 30ms, FA 90deg, 39 axial slices, 3mm thick, 0.75mm interval, matrix 64x64, FOV 192mm, BW 1420 Hz/Pix, 140 volumes) on a 3T MRI scanner. As anatomical references, T1 and T2 weighted images were obtained. The functional images were preprocessed by using SPM12 (UCL, London). The RSN activation was extracted by GIFT4.0 (UNM, NM). Twenty-five independent components (ICs) obtained were assigned by Shirer’s template [6]. Back reconstructions of each subjects’ contrast maps were analyzed using the statistical tool of SPM12 to estimate the effects of their participation in PECs and the scores of behavioral data on the RSN activation.Results
Among the 75 subjects, 44 participated in the PECs (179.09±203.26 months). In all subjects, the exercise time per day was 90 minutes, and once a week throughout a year. The MMSE scores were over 27 in all subjects except one subject (24). The effects of the history in PECs and PA on the RSN activations were evaluated by one sample t-test with a covariate of total sum of their participation in PECs (months). The following regions were detected as the effects of interest by this regressor (k>10, p<0.01, uncorrected). Positive correlations with the total months of participation in the PECs were detected in the dorsal DMN (MNI coordinate = [2 46 16], T = 3.42), the ventral DMN ([-28 16 50], T = 3.83), the anterior salience network (SN) ([-30 40 30], T=4.11), posterior SN ([16 -52 64], T = 4.64), basal ganglia network (BGN)([-8 6 4], T=3.57), the visuospatial network (VSN) ([-36 -40 42], T=4.34) and the posterior SN ([-16 -54 64], T=4.93). Negative correlations were detected in the VSN ([32 -54 56], T=6.85) and the anterior cingulate gyrus (ACG) region of the anterior SN ([-8 30 22], T=3.12). Activation in the ACC was also negatively correlated with the scales of IPAQ-A ([-8 28 24], T=3.41; [8 36 18], T = 3.31) and IPAQ-E ([-8 12 38], T= 3.15).Discussion
It was demonstrated that long-term participation in PECs augments the activations in the DMN, SN, BGN and VSN. The activation in the DMN is declined depending on age [7] and increased by aerobic PE for mid-term period [8]. In contrast, activation in the SN is augmented in normal aging [9] suggesting more demand for perception of the surrounding environment and integration of the sensory information. In this study, the activation in the ACG was decreased depending on the longer history of participation in PECs as well as higher physical activity evaluated by IPAQ A and E scales. These findings agree with the previous reports. It was suggested that this decrease in healthy older adults may represent optimization of salience processing rather than its functional declination, i.e. less age-related augmentation of cognitive demand for sensory integration. We hypothesize that both recovery and sedation of the activity in the RSNs against the age-related change may be the biomarker to evaluate the effects of long-term physical interventions.Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (KAKENHI) #15H03104 supported by MEXT. We thank to Hisako Sato BA from the Handa City Association for Health Promotion, Aichi Prefecture, Japan, for supporting the measurement sessions.References
[1] Colcombe S et al., Psychological Science 14, 125-130, 2003 [2] Spartano NL et al., Neurology 86, 1313-1319, 2016 [3] Andel R et al., MEDICAL SCIENCES 63, 62-66, 2008 [4] Sorg C et al., PNAS 104, 18760-18765, 2007 [5] Petrella JR et al., Neurology 76, 511-517. 2011 [6] Shirer WR et al., Cerebral Cortex 22, 158-65, 2012 [7] Vidal-Pineiro D et al., Front Aging Neurosci 6, pii 256, 1-17, 2014, doi: 10.3389/fnagi.2014.00256 [8] Voss MW et al., Front Aging Neurosci 2, pii 32, 1-17, 2010, doi: 10.3389/fnagi.2010.00032 [9] Chen SHA et al., IEEE Proceedings, ISMICT 2013, 218-222, 2013, doi: 10.1109/ISMICT.2013.6521732Figures
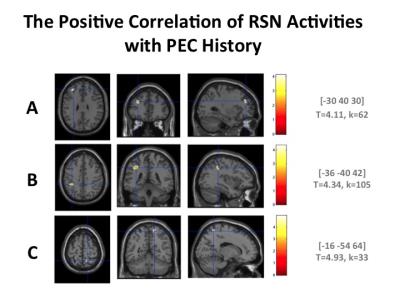
Figure 1
RSN activities
correlating with the history of participation in PECs. The total months of
participation was used as the covariate. Positive correlations in the anterior
SN (A), VSN (B) and posterior SN (C) were detected (k > 10,
p <.01, uncorrected).
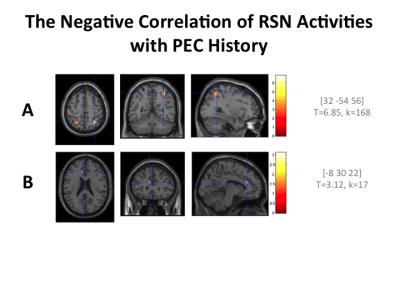
Figure 2
RSN
activities negatively correlating with the history of participation in PECs. The
total months of participation was used as the covariate. Negative correlations
in the VSN (A) and anterior SN (B) were detected (k > 10, p
<.01, uncorrected).